Computer tomography guided lung biopsy using interactive breath-hold control: a randomized study
Introduction
The advancements in computed tomography (CT) have had a major influence on the detection of cancer-suspected lung nodules (1). Both smaller and increasing numbers of nodules can now be detected because of improvement in the scan resolution (2,3). The diagnostic workup of these nodules is a challenge, since the distinction between malignant and benign nodules is essential for treatment planning (4). CT-guided lung biopsy is a minimally invasive method to obtain tissue diagnosis of small indeterminate pulmonary nodules (5-7). However, the accuracy of the biopsies is a key issue, since false positive and false negative biopsies have a huge impact on the treatment and prognosis of the patient (8).
Biopsy of small nodules is often hampered by respiration motion, which causes the nodule to move during the biopsy procedure (6). It is presumed, that during normal breathing, structures in the lung may move up to 6 cm. Even if specific breath hold instructions are given to the patient, it is difficult to reproduce a consistent level inspiration and expiration.
An interactive bellows-based breath-hold control (IBC) system has been developed in order to guide the patient to keep a constant level of breathing by giving feedback (9). The system registers changes in the thoracic girth, which is closely correlated to diaphragm motion and the changes in the lung parenchyma. Bellows-based systems have proven to decrease in the variability of the position of the diaphragm (9) and increase depiction of mobile target nodules when using CT fluoroscopy (10).
Real-time and intermittent (quick-time) CT fluoroscopy-guided lung biopsy has its advantages, since live images may help to determine more precisely where the nodule is located during the procedure (11). Unfortunately, high radiation exposure to both the patient and the operator argues against this method as a routine. An alternative, which decreases the risk of radiation, is to perform the lung biopsy using stepwise CT with laser-guided puncture (12,13), which, however, has the disadvantage of depriving the operator of real-time guided biopsy. In this context, we decided to investigate the use of IBC in connection with stepwise CT-guided lung biopsy with laser-guided puncture.
Aim of the study
The aim of this study was to investigate if IBC used in stepwise CT-guided lung biopsy with laser-guided puncture improves biopsy accuracy and decreases the risk of complications. The main purpose was to perform an evaluation of the interactive breath hold control on the clinical handling of the patients.
Methods
Consecutive patients with indication of CT-guided lung biopsy admitted in the period from March 1, 2010, to February 29, 2012, were included (N=407). Exclusion criteria were poor pulmonary function test with a forced expired volume in the first second (FEV1) <0.5 liter, abnormal coagulations status i.e., INR >1.5, platelet count less than 100,000 per mL, and ECG with sign of recent myocardial infarction or serious arrhythmias. Patients unwilling or unable to participate were excluded.
Previous studies have shown an accuracy between 0.80–0.83 (14,15) for CT guided lung biopsies. We considered a 10–15% increase in accuracy as clinically significant. With a power (β) of 20% and with a significance (α) of 5% the sample size must be at least 107 in each group (accuracy of 93%). We planned for over 400 participants in total because of relative high number of dropouts in especially the plus IBC group.
After oral and written informed consent, the patients were randomized to CT-guided lung biopsy with (plus IBC) or without the use of IBC (minus IBC). Demographic data, pathology report of tissue analyses, and the complications were recorded. The CT-guided lung biopsies were performed by four pulmonologists (authors MN, VM, AN, PFC) and one radiologist (PSM). The clinical data including results of the biopsies and complications were collected in cooperation between a pulmonologist (author SK-A) and a medical student with specific knowledge of the handling of databases (author TMH). The assessment of the results in an anonymous form was done by the pulmonologist (author SK-A), who was blinded with regards to the randomization.
Of obvious reasons the accuracy of the biopsies could not be assessed by operating the patients as a routine. Since the aim of the study was to assess the use of the interactive breath hold control in daily clinical practice a conclusive biopsy was defined as a biopsy being found to be of crucial clinical importance for the planning of the strategy for the patient.
Ethical considerations
The study was approved by the Danish National Committee on Biomedical Research Ethics (ref. No.: H-4-2010-fsp 1). The study was registered at ClinicalTrials.org (NCT01236937).
The breath-monitoring system
The bellows-based breath hold system (IBC, Mayo Clinic Medical Devices, USA) consists of two elements: a belt which is strapped around the thorax of the patient and a voltage-controlled light unit with eight LEDs. Belt stretch from respiratory motion is converted into voltage readings and displayed on the light unit giving biofeedback to the patient, since different lights are activated depending on the depth of the respiration. The breathing of the patient was monitored by observing the movements of the LED lights. With the help of the lights, the patients can be instructed to keep their respiration constant at a certain depth during the biopsy procedure.
Laser-guided stepwise CT lung biopsy
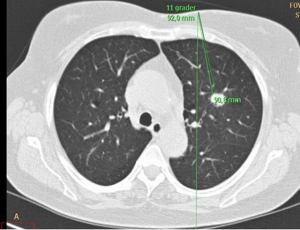
The patient was placed in the CT scanner partly enclosed by a vacuum blanket to prevent movements. Intravenous access was established, but no sedation was given. The target was identified by performing CT, whereupon an optimal route (puncture site and angle) for the guide needle and an optimal position for the patient were decided (Figure 1). If necessary, the position of the patient was changed and a new CT performed. The aims were to have the shortest possible distance to the target, to be free of bone structures, to have a perpendicular passage of the pleura, to avoid passing pleura more than one time, and to keep maximal distance to vessels. With the patient outside the CT scanner, the coaxial guide needle was then inserted under the use of local anesthesia and with guidance of the laser system. Then, CT was repeated to ensure the correct position of the guide needle in the target. Then, the patient was again placed outside the CT scanner, and the biopsy needle (18G-15-10 or 18G-9-10) was inserted through the guide needle, whereupon the biopsies were taken. After the biopsy procedure, CT was repeated to look for pneumothorax and other complications by the doctor who had the clinical responsibility for the patient and by the doctor on call that day. After 1–2 hours, an X-ray of the chest was performed in two views (anterior-posterior and lateral).
Definitions and statistics
The “needle time” was defined as the time in minutes from inserting the guidance needle until it was removed. The total procedure time was defined as the time in minutes from placing the patient in the CT scanner until the patient left the CT scanner. Pneumothorax was defined as visible air in the pleural cavity based on CT or X-ray of the chest. A conclusive biopsy was defined as a tissue sample that was found to be of crucial importance for planning the further strategy for the patient. An inconclusive biopsy was defined as a biopsy leading to re-biopsy or operation of diagnostic purposes. Quantitative data and complication rate in the two groups were compared using a t-test and chi square test. Predictors for pneumothorax were analyzed using both univariate and multivariate logistic regression analyses. R statistical software version 2.7.1 was used for the statistical analyses, P values equal to or less than 0.05 were considered statistically significant.
Results
Of the 407 patients included, 201 patients were randomized to the use of IBC (plus IBC), and in 206 patients the use was omitted (minus IBC).
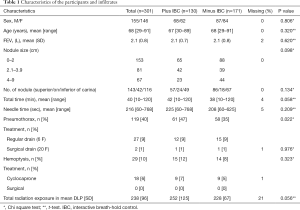
In 71 patients in the first group, the use of the belt was cancelled and the patient excluded from the study. In 15 of these 71 cases the patient did not understand the use of the IBC; in 9 cases the belt was in the way of the needle; in 3 cases the patients could not see the light diodes; in 1 case the belt was too short, and one patient found the belt uncomfortable; and in 1 case the sensors did not function (n=30). In the rest of the 71 cases the reasons were independent of the belt, for example pneumothorax, claustrophobia, pain, that the target was hidden behind bone structures (16 cases), or regression of the infiltrate (11 cases) compared with the CT leading to admission for biopsy (n=41). In the group randomized to minus IBC, 35 patients were excluded for reasons similar to the reasons mentioned for the 41 patients. A significantly higher number of patients (71 versus 35, P=0.00) was excluded from the group randomized to plus IBC than randomized to minus IBC. The remaining 301 patients were evaluated as shown in Table 1.
Full table
We found a higher rate of pneumothorax in the plus-IBC group (47%) versus minus IBC (35%) using univariate analyses (P=0.03). Borderline higher total procedure time and radiation (P=0.06) were found in the plus-IBC group. No other significant differences were found in participants and infiltrates characteristics (Table 1).
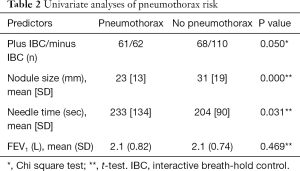
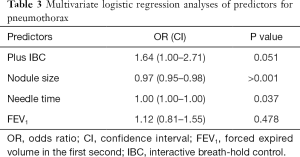
Univariate analyses showed in the plus-IBC group that smaller nodules and longer needle time were associated with pneumothorax (Table 2). This was confirmed in the multivariate logistic regression analyses of predictors for pneumothorax, which showed close to significant P value for randomization to plus IBC [odds ratio (OR) 1.6, P=0.051]. In the multivariate analyses, longer needle time and smaller size of the target were significant predictors for pneumothorax, with a low OR near 1 (Table 3). FEV1 was not correlated to the risk of pneumothorax. Of the patients with pneumothorax, approximately 24% (29/119) were treated with a pleural chest tube (Table 1). All patients requiring a chest tube and all patients with hemoptysis were hospitalized for observation.
Full table
Full table
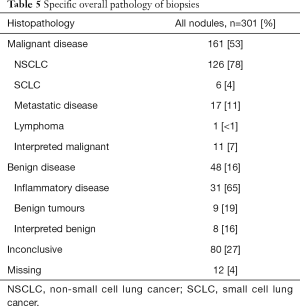
The number of inconclusive biopsies did not differ between the groups randomized to plus or minus IBC (Table 4). Overall, approximately 53% of the biopsies showed malignancy, approximately 16% were benign, and approximately 27% were inconclusive (Table 5). We found an accuracy rate (true positive + true negative/all cases) of 68% in the plus-IBC group and 71% in the minus-IBC group (P=0.567, chi sqr). The accuracy of the biopsy was independent of the location of the biopsy target (superior or inferior to the carina) in both univariate and multivariate analyses (data not shown).

Full table
Full table
Discussion
In other studies, the IBC system has shown results pointing in the direction of improvement of biopsy quality and reduction in the complication rate (9-11). However, in our randomized prospective study with more than 400 patients, we could not confirm these results. The number of inconclusive biopsies was the same in both groups, and the risk of pneumothorax was increased in the IBC group (47% versus 35%). To our knowledge this has not been demonstrated before. One explanation for this could be because of patient tend to perform Valsalva when asked to hold their breath at a certain point in the respiration cycle and thereby increasing the intrathoracic pressure which may increase the risk of pneumothorax. Patients without monitoring are not forced to hold their breath at a certain point and therefore may have relatively lower intrathoracic pressure.
The pneumothorax risk overall was relatively high compared to other studies (16,17), which in part can be explained by the fact that any visible sign of air in the pleural cavity on CT was defined as pneumothorax. Only 10% of all patients required pleural drainage. We found an overall accuracy of 69% (209/301), which is relatively low, but it may in part be explained by a relatively large portion of difficult patients with small lung infiltrates. However, the accuracy was the same in the two groups, and the absolute figures, therefore, do not affect the overall conclusion. We would have expected that the patients randomized to plus IBC would have shown a longer procedure time, since it can be time consuming to place the belt, but this was not the case. An explanation could be that the belt had to be removed again in 34% of the patients (71 out of 206), which saved time. This fact also shows that in a relatively large number of patients the belt could have been omitted from the beginning of the procedure.
Theoretically, it could also be expected that the possible advantage of IBC would be greater in selected subgroups with small nodules, but this was not found. Longer needle time and small nodule size predicted pneumothorax in both the univariate and multivariate analyses. Even though the OR was low, the results were significant.
A limitation of the study is that we were not able to calculate a precise risk of overlooking a possible advantage of IBC, but it seems unlikely that the inclusion of more patients would have changed the overall conclusion.
A strength of the study in comparison with other studies is that we carefully explain the difficulties in using the IBC instead of excluding cases where the use of the belt failed. Also, we describe the use of IBC in consecutive unselected patients, this approach reflects everyday clinical practice, which is the scenario of interest to the reader.
Conclusions
Based on our findings, we cannot recommend the use of IBC in unselected consecutive patients referred for laser-guided stepwise CT lung biopsy in a clinical setting like ours, since the technique increased the risk of pneumothorax without decreasing the risk of inconclusive biopsies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Danish National Committee on Biomedical Research Ethics (ref. No.: H-4-2010-fsp 1). The study was registered at ClinicalTrials.org (NCT01236937).
References
- Verschakelen JA, Bogaert J, De Wever W. Computed tomography in staging for lung cancer. Eur Respir J Suppl 2002;35:40s-8s. [Crossref] [PubMed]
- Costello P, Anderson W, Blume D. Pulmonary nodule: evaluation with spiral volumetric CT. Radiology 1991;179:875-6. [Crossref] [PubMed]
- Benjamin MS, Drucker EA, McLoud TC, et al. Small pulmonary nodules: detection at chest CT and outcome. Radiology 2003;226:489-93. [Crossref] [PubMed]
- Ashraf H, Dirksen A, Loft A, et al. Combined use of positron emission tomography and volume doubling time in lung cancer screening with low-dose CT scanning. Thorax 2011;66:315-9. [Crossref] [PubMed]
- Quint LE, Kretschmer M, Chang A, et al. CT-guided thoracic core biopsies: value of a negative result. Cancer Imaging 2006;6:163-7. [Crossref] [PubMed]
- Tsai IC, Tsai WL, Chen MC, et al. CT-guided core biopsy of lung lesions: a primer. AJR Am J Roentgenol 2009;193:1228-35. [Crossref] [PubMed]
- Poulou LS, Tsagouli P, Ziakas PD, et al. Computed tomography-guided needle aspiration and biopsy of pulmonary lesions: a single-center experience in 1000 patients. Acta Radiol 2013;54:640-5. [Crossref] [PubMed]
- Gelbman BD, Cham MD, Kim W, et al. Radiographic and clinical characterization of false negative results from CT-guided needle biopsies of lung nodules. J Thorac Oncol 2012;7:815-20. [Crossref] [PubMed]
- Carlson SK, Felmlee JP, Bender CE, et al. CT fluoroscopy-guided biopsy of the lung or upper abdomen with a breath-hold monitoring and feedback system: a prospective randomized controlled clinical trial. Radiology 2005;237:701-8. [Crossref] [PubMed]
- Locklin JK, Yanof J, Luk A, et al. Respiratory biofeedback during CT-guided procedures. J Vasc Interv Radiol 2007;18:749-55. [Crossref] [PubMed]
- Carlson SK, Felmlee JP, Bender CE, et al. Intermittent-mode CT fluoroscopy-guided biopsy of the lung or upper abdomen with breath-hold monitoring and feedback: system development and feasibility. Radiology 2003;229:906-12. [Crossref] [PubMed]
- Nitta N, Takahashi M, Tanaka T, et al. Laser-guided computed tomography puncture system: simulation experiments using artificial phantom lesions and preliminary clinical experience. Radiat Med 2007;25:187-93. [Crossref] [PubMed]
- Kim GR, Hur J, Lee SM, et al. CT fluoroscopy-guided lung biopsy versus conventional CT-guided lung biopsy: a prospective controlled study to assess radiation doses and diagnostic performance. Eur Radiol 2011;21:232-9. [Crossref] [PubMed]
- Tsukada H, Satou T, Iwashima A, et al. Diagnostic accuracy of CT-guided automated needle biopsy of lung nodules. AJR Am J Roentgenol 2000;175:239-43. [Crossref] [PubMed]
- Priola AM, Priola SM, Cataldi A, et al. Diagnostic accuracy and complication rate of CT-guided fine needle aspiration biopsy of lung lesions: a study based on the experience of the cytopathologist. Acta Radiol 2010;51:527-33. [Crossref] [PubMed]
- Boskovic T, Stanic J, Pena-Karan S, et al. Pneumothorax after transthoracic needle biopsy of lung lesions under CT guidance. J Thorac Dis 2014;6 Suppl 1:S99-S107. [PubMed]
- Wu CC, Maher MM, Shepard JA. Complications of CT-guided percutaneous needle biopsy of the chest: prevention and management. AJR Am J Roentgenol 2011;196:W678-82. [PubMed]


