Ethical challenges involved in obtaining consent for research from patients hospitalized in the intensive care unit
Introduction
Clinical research is the cornerstone of medical progress, and is fundamental to advancing our knowledge of the epidemiology, diagnosis, treatment and outcomes of disease. After the high-profile Nuremberg trials that took place in the wake of World War II, a number of landmark documents regarding the ethics of clinical research were published, the most widely known among these being the Nuremburg Code (1) and the Declaration of Helsinki (2). These seminal documents outline the basic tenets to be respected in performing research in human subjects, and foremost among these principles is the precept that every person has the right to determine what happens to his/her own body, and as such, any person participating in research must do so voluntarily, after receiving sufficient pertinent information, and without being constrained to do so. This voluntary participation is enshrined in the form of written informed consent, generally materialized in a consent form that is signed by both the physician and the patient before the initiation of any procedures relating to the research (Figure 1). To a large extent, the same principles apply to standard care procedures, where the patient is equally entitled to clear and transparent information about the procedures, risks, benefits and possible alternatives.
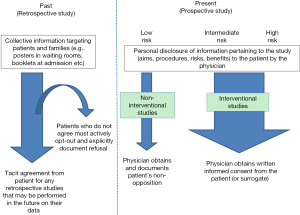
Therein lies the rub in the case of research performed in the field of critical care. Patients who are admitted to the intensive care unit (ICU) very often lack decisional capacity, either because of the illness that caused them to be admitted (e.g., pain, trauma, shock, coma), or because they are sedated (opiates, mechanical ventilation etc.). Studies of research practices involving critically ill patients report that only around 10% of patients admitted to the ICU possess decisional capacity (3,4). So, when faced with a patient who is decisionally incapable, how is the critical care physician supposed to proceed if he/she wishes to enrol that patient in clinical research? Including critically ill patients in medical research raises many ethical challenges, which we will attempt to review here. In some ways, the challenges of this situation overlap to a large extent with those of several other domains, such as end-of-life research, research in paediatric or neonatal patients, genetic research, and organ donation. However, since each particular context has its own defining characteristics linked to the age of the patients involved, their likely prognosis, or their likelihood of recovery, for example, we intend to focus exclusively on the problem of obtaining consent for clinical research in critically ill patients, and will not address the other areas where similar issues may arise. In the same way, the ethical issues involved in obtaining consent for routine care, vaccination and screening programmes are also not addressed in this review, since the stakes and the patient population are different from the context of clinical research.
Decisional incapacity
Generally speaking, in relation to the provision of informed consent, decisional capacity could be considered to cover the ability to receive and process relevant factual information pertaining to the study’s aims, potential risks, benefits, and procedures; to appreciate one’s own situation and likely outlook, and then to consciously decide whether or not to participate in the study in light of one’s underlying morals and values (5,6). Once the patient has decided to commit to one course of action, he/she must be able to communicate this decision to the entourage and in particular, to the physician requesting the consent, either by speaking, writing, use of sign language or some other form of communication (5). This whole process assumes not only that the patient understands what he/she is authorizing, but also that he/she be aware of the very fact that he/she is authorizing something (6). Several conditions may hamper the patient’s decisional capacity, not least among these being the medical condition that caused the patient to be hospitalised in the ICU, including trauma, shock, or other life-threatening clinical conditions. In one analysis of a total of 226,942 consecutive admissions to 97 ICUs in the USA, the rate of patients under mechanical ventilation ranged from 20.7% to 38.9% (7). Extreme pain, which may be exacerbated by care procedures (8), combined with anxiety and fear of imminent death may also cloud the patient’s judgement, and render them unreceptive to information and unwilling to consider consenting to trial participation. Indeed, 20% to 45% of ICU survivors report negative memories of their ICU stay, including memories of pain, at 3 months after discharge (9). Finally, a large proportion of patients arriving at the ICU may be sedated (10-12), which obviously precludes any possibility of obtaining consent first-hand.
Apart from these obvious conditions of decisional incapacity, other situations may arise where the ethical challenges may be less evident. For example, in patients who are judged to have decisional capacity and who are approached for consent, the consent forms that are given to the patient to be signed may be long and complicated, and hard to understand for patients who are already trying to come to terms with experiencing a sudden and often life-threatening health event. Indeed, consent forms vary in length between trials and centres, and may range from 2 to 10 pages even within a same trial (13). One study of informed consent procedures in the context of clinical emergency (i.e., myocardial infarction) indicated that there was a mismatch between the level of education of the study participants and the level of education required to comprehend the informed consent form (14). Similarly, there may be a mismatch between the provider of the information (i.e., the physician) and the patient, such that the communication fails to achieve its objective of relaying important factual information to the patient about the study so that they may make a decision about participation. Indeed, it is important that the study documentation and the information provided by the physician or healthcare team be comprehensible for all patients, regardless of their socio-economic background.
Lastly, delirium may be present in up to one third of patients in the ICU (15,16), and ICU physicians reportedly recognize less than one third of delirious critically ill patients when they are not using an instrument to aid in their diagnosis (17). Furthermore, in an observational study among patients admitted to the ICU, judged to be competent (Glasgow coma scale score of 15, fully oriented and free of mechanical ventilation) and who accepted to participate in a research study, 80% of the patients recognized 10 to 12 days after informed consent had been obtained that they had accepted to participate in a clinical trial, but only 32% could recall the purpose of the trial and its related risks (18). The same authors also showed even lower recall rates in another study in the ICU setting (18). In these conditions, some ICU patients may be approached for consent to research on the understanding that they are competent, whereas in actual fact, they may have undetected delirium, cognitive impairment or poor recall, and consequently, reduced decision-making capacity.
Therefore, while in some situations, it is clear that the ICU patient is decisionally incompetent, such as in cases of trauma, shock or sedation, there are other situations where the integrity of the informed consent process is likely to be jeopardized, even though the patient may be thought to be competent. Particular caution is therefore required to ensure that the underlying ethical principles of informed consent are adequately respected.
Surrogate decision makers (SDMs)
In the event that the ICU patient is unable to provide consent first-hand, the most widely adopted approach is to obtain consent (or assent) for research opportunities from a member of the patient’s family or entourage on the patient’s behalf; this person is usually termed the SDM. This is the preferred strategy for enrolment for most stakeholders in research, including ICU survivors, family members, ethical review boards and the public (13). In one prospective, observational study of all critically ill adults eligible to participate in research studies at 23 Canadian ICUs, it was reported that out of 452 eligibility events, SDMs were involved in over 90% of consent encounters, with patients deciding for themselves in only 8.9% of all encounters (3). However, obtaining assent from SDMs is beset with its own lot of ethical challenges.
Firstly, SDMs are often suffering from high stress levels created by the sudden admission of a loved one to the ICU. They may be anxious and afraid of losing someone close to them, and they can find the ICU environment frightening. Information overload is often a problem, since they are continually receiving updates of their loved-one’s state of health, usually on medical problems of which they have little understanding. They may be overwhelmed by the circumstances and their emotions, and thus unlikely to be able to think clearly enough about the pros and cons of research participation. High stress levels among family members have previously been reported, and up to two thirds of family members of patients hospitalized in the ICU may suffer anxiety or depression (19-21). This has led certain authors to posit that this emotional suffering should be taken into consideration when asking family members to make important health decisions for their loved one, since their emotional suffering may impair their understanding, or their capacity to evaluate the benefits and risks associated with the proposed research (21). It has also been shown that these conditions of stress are among the predominant reasons why SDM refuse to assent to research on behalf of their loved one (22,23).
A further difficulty in obtaining assent from SDMs can even be the identification of the person who is best placed to act as surrogate. Indeed, procedures exist in many countries for naming a designated official surrogate, such as by delegating official power of attorney for healthcare, or by enshrining one’s desires in the form of written advance directives, which represent a “living will” specifying the types of life-sustaining procedures and treatments one would like to have (or not) in case of decisional incapacity. However, the proportion of patients who have established advance directives is low, ranging from 26.3% in one study of 7,946 US adults participating in a health survey designed to be representative of the US population (24), to 42.4% in a study of 450 critically ill older adults requiring mechanical ventilation and admitted to the ICU (25), although recent evidence suggests that the rate of prevalence of advance directives is on the rise (26,27). Nonetheless, when no official surrogate has been designated by the patient, the care-giving team in the ICU may have difficulty identifying a suitable person in the patient’s entourage with whom to interact, or from whom to request consent for research, for example. Indeed, choosing the spouse, which is often the default position, may not be the most suitable choice in the patient’s view (28,29). Several characteristics among the persons attending the patient may help the physicians and ICU staff to identify the most suitable surrogate, such as knowledge of the patient’s wishes, the nature of their bond with the patient, and an adequate level of understanding (30).
Once a suitable surrogate person has been identified, and deemed capable of making decisions on behalf of the patient, there is clear evidence that their choices are not always in line with what the patient actually would have wanted. For example, in a systematic review of 16 studies, involving 151 hypothetical scenarios and 2,595 surrogate-patient pairs, and collectively analyzing 19,526 patient-surrogate paired responses, the overall accuracy of SDMs for predicting patients' treatment preferences was 68% (31). In a study by Coppolino et al., patients agreed or declined to provide informed consent to two hypothetical research trials (one representing minimal risk and the other trial greater than minimal risk), and surrogates subsequently attempted to predict the patients’ responses (32). The authors reported overall surrogate positive predictive value for the low-risk study at 84.0% and for the high-risk study at 79.7%, resulting in false-positive consent rates of 16% to 20.3% if SDMs had been making the consent decisions. Similarly, Ciroldi et al. conducted a prospective multicenter study in ten ICUs in which two hypothetical studies were simultaneously submitted to the patient, surrogate, and physician at the time that the patient was discharged to a ward (33). The authors observed patient-surrogate discrepancy in 32% of cases in the minimal-risk study, and 42% discrepancy in the greater-than-minimal risk study, with SDMs underestimating the patient’s wish to participate (33).
Finally, when obtaining assent from families for inclusion of a critically ill patient in research, it is important for the physician’s communication to be totally transparent and clear. Many families or SDMs may be unaware that research can be or is being carried out in the ICU (34), and in the stress of the situation, may mistakenly interpret information about clinical trial participation to equate with opportunities for care. This phenomenon is commonly known as the “therapeutic misconception”, and was first described by Appelbaum and colleagues in 1982 who observed through a series of interviews with patients with psychiatric disorders that many were unable to distinguish between clinical research and medical care (35,36). A similar misconception is the perception by the family that their loved one will receive better care if enrolled in the clinical trial, and they may “inaccurately attribute therapeutic intent to research procedures” (37). While there is some evidence that hospitals or individual units of a hospital with a strong research culture may provide better quality of care (38,39), the posit that patients included in a trial will receive more attention or better care is fundamentally incompatible with the basic principles of research. It is every physician’s duty to ensure optimal management for every patient with the gold-standard of care, regardless of whether they accept or decline to participate in research. Therefore, the message delivered by the physician who approaches SDMs for assent must be crystal clear about the experimental nature of the opportunity being proposed.
Time-critical research
Despite data indicating that SDM decisions may not fully coincide with the patient’s actual wishes (were the patients able to answer for themselves), many research protocols require inclusion procedures to be performed (and thus, consent to be obtained) within a specific and often short time window. In these circumstances, if the patient is decisionally incapacitated and the family members are not receptive to the idea of research participation while they are in the throes of emotional turmoil at the sudden ICU admission of their loved one, opportunities for research may be lost if no consent (or assent) can be obtained in a timely manner. In a prospective observational study of research recruitment practices in 23 adult ICUs across Canada, Burns et al reported that in 130 of 452 (28.8%) eligibility events, consent was missed, and in a further 129 of 452 (28.5%), consent could not be obtained for operational reasons (3).
Mortality rates are high among patients admitted to the ICU, and this can pose additional problems for research procedures in this patient population. Even when assent can be obtained for research, a high proportion of patients may subsequently die after inclusion, with the result that retrospective consent cannot be obtained. Furthermore, among survivors, a certain proportion may never regain decisional competence. Harvey et al performed a descriptive study nested within the randomized PacMan trial, among 56 ICUs in the UK, and observed high mortality (60.6%) and low first-hand consent (13/498, 2.6%). In addition, among the 188 (39.4%) survivors, 175 (93.1%) gave retrospective informed consent, while 6 (3.2%) refused, and 7 (3.7%) did not regain mental competency. Interestingly, in this study, the 7 patients who were included but did not regain decisional capacity were included in the final analysis. Indeed, Jansen et al. have purported that excluding patients from analysis because they retrospectively refuse consent, or die before consent can be obtained, even though they have already been included and the study procedures performed, can introduce significant bias into the results (40,41). Similarly, systematic exclusion of certain types of patients on the basis that consent cannot be obtained can also bias the results, if those excluded (or non-consenting patients) are systematically different from those who are enrolled. This greatly impairs the “generalizability” of the results, even if the required sample size is successfully accrued, and can hamper comparison between studies.
Alternative consent procedures
The use of alternative procedures for obtaining consent, such as waived or deferred consent, could help to enhance enrolment in time-sensitive situations (42,43). Annane et al. showed that waiver of consent increased inclusions from 4 to 10 patients per month in one trial in critical care, allowing successful completion of the trial within the planned timeframe (42). In a study from the MRC CRASH Trial, an international randomised controlled trial of corticosteroids in head injury, it was found that time from injury to randomisation was significantly reduced (1.2 hours, 95% CI: 0.7 to 1.8 hours) and patient recruitment was higher in hospitals where consent was waived compared with those that required relatives consent (44). Zelen’s design, first published in 1979, is an alternative method of randomization whereby only patients allocated to the intervention arm are approached for consent, on the basis that the non-intervention group are receiving standard care, which they would have received anyway (45). This method presents the advantage that the patient knows their treatment allocation group before providing consent. Deferred consent is yet another option, whereby the study procedures are initiated as soon as possible without consent, and written consent is sought from the patient or SDM as soon as possible. Deferred consent models have successfully been used in several emergency trials (46,47), although they pose the problem of what to do with the data from patients who die before consent can be obtained (40,41), as mentioned earlier. In addition, ethics committees and/or institutional review boards (IRBs) may be loathe to approve protocols where consent is deferred or waived.
Legislation regarding informed consent
Current Food and Drug Administration (FDA) regulations allow for IRBs to give approval for research to proceed without obtaining informed consent from all participants, provided that a certain number of conditions are met and documented, including, for example, that the patient must be in a life-threatening situation; obtaining consent is not feasible; there is a chance of direct benefit for the participant; and the research cannot reasonably be carried out without the waiver, amongst other conditions (48). In Europe, the situation is slightly different, with variations in practices between different countries. The European Directive 2001/20/EC relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use introduced in 2001 (49) stipulated that special protection should be afforded to vulnerable patients, namely those unable to decide for themselves. The very restrictive conditions outlined regarding incapacitated adults raised concerns in the research community that the directive might in fact lead to a reduced number of patients being included in trials, thus stymieing research within the European Community (50,51). In practice, the level of transposition of this EU directive into national laws through the European Union was variable, and the national legislation of some countries was more flexible on this particular point (52). A new clinical trials legislation was adopted on 16 April 2014 by the European Parliament and entered into force on 16 June 2014, but is still in a period of transition towards full application across the 27 member states of the European Union (53). This new legislation aims to simply harmonize the implementation procedures for clinical research across Europe, but concerns persist among researchers regarding the dispositions for emergency situations and obtention of consent (52,54). It remains to be seen how this new legislation is implemented and taken up into national legislation.
Re-consenting
Re-consenting is another possible approach for obtaining consent for research from critically ill patients who may be decisionally incapacitated at admission, or for whom no assent could be obtained due to a failure to contact a family member or SDM. Re-consenting involves assessing the decision-making capacity of the patient periodically during the hospital stay to evaluate whether competence is regained before discharge, or later. In practice, however, it is not always feasible to perform regular screening for recovery of decisional capacity due to the already considerable workload of ICU staff, and this therefore precludes the possibility of obtaining informed consent directly from the patient. In an analysis of 1,164 patients enrolled into three Acute Respiratory Distress Syndrome Network trials, Smart et al. investigated surrogate consent and re-consenting for genetic studies and found that among patients who survived and regained decisional capacity sufficient to provide re-consent, 522 of 539 (97%, 95% CI: 96–98%) affirmed their study participation (55). Similarly, in a study of 240 capable and consenting survivors of critical illness, Scales et al. reported that more than three-quarters (76%) of patients selected “consent by substitute prior to enrolment” as their preferred framework for inclusion in a clinical trial (56). However, while evidence suggests that few patients refuse consent retrospectively (43), there is no existing guidance in the literature or ethical frameworks about how long attempts to re-consent should be continued (13).
To circumvent this difficulty, some authors have called for the use of research methodology other than randomized clinical trials, or more specifically, wider acceptance (and publication) of research that is less highly ranked on the scale of methodological virtue (57,58). One of these authors has contended elsewhere (59) that randomized clinical trials may not be the “be all and end all” of research methods, and that their importance has been overemphasized, thus opening the door to other forms of research into unanswered clinical questions in the field of critical care.
Conclusions
Clinical research remains a vital contributor to medical knowledge, and is an established and integral part of the practice of medicine worldwide. Respect for patient autonomy and ethical principles dictates that informed consent must be obtained from subjects before they can be enrolled into clinical research, yet these conditions may be difficult to apply in real practice in the ICU. A number of factors serve to complexify the consent process in this setting, first among these being incapacitation of the patient due to illness or sedation. In patients who are unable to make decisions themselves, consent for research may be obtained from a designated proxy or family member, commonly termed a “surrogate decision maker”. However, SDMs who are trying to deal with the emotional, psychological and logistic impact of a sudden hospitalisation of their loved-one are not always open to the idea of research or emotionally equipped to reflect rationally on the opportunities being proposed to them. In addition, time constraints and workload pressures on the attending physician may render consent opportunities unfeasible, and the resulting loss of eligible patients could represent a bias in clinical trials, or limit the generalizability of their results. Alternative procedures such as deferred or waived consent have been used in the past and may be suitable alternatives in certain conditions, provided appropriate approval from IRBs can be obtained, in accordance with existing legislation. Many of the questions inherent to the conduct of clinical research in critically ill patients remain debated. Indeed, more than 50 years after Henry Beecher’s influential paper addressing the dilemmas of human experimentation (60), the controversy rages on, and will likely provide food for thought in the medical literature for many years to come.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Tribunal Nuremberg Military. The Nuremberg Code. JAMA 1996;276:1691. [Crossref] [PubMed]
- World Medical Association. Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. Helsinki 2013. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/
- Burns KE, Zubrinich C, Tan W, et al. Research recruitment practices and critically ill patients. A multicenter, cross-sectional study (the Consent Study). Am J Respir Crit Care Med 2013;187:1212-8. [Crossref] [PubMed]
- Smith OM, McDonald E, Zytaruk N, et al. Rates and determinants of informed consent: a case study of an international thromboprophylaxis trial. J Crit Care 2013;28:28-39. [Crossref] [PubMed]
- Rincon F, Lee K. Ethical considerations in consenting critically ill patients for bedside clinical care and research. J Intensive Care Med 2015;30:141-50. [Crossref] [PubMed]
- Silverman HJ. Ethical considerations of ensuring an informed and autonomous consent in research involving critically ill patients. Am J Respir Crit Care Med 1996;154:582-6. [Crossref] [PubMed]
- Wunsch H, Wagner J, Herlim M, et al. ICU occupancy and mechanical ventilator use in the United States. Crit Care Med 2013;41:2712-9. [Crossref] [PubMed]
- Ayasrah S. Care-related pain in critically ill mechanically ventilated patients. Anaesth Intensive Care 2016;44:458-65. [PubMed]
- Chahraoui K, Laurent A, Bioy A, et al. Psychological experience of patients 3 months after a stay in the intensive care unit: A descriptive and qualitative study. J Crit Care 2015;30:599-605. [Crossref] [PubMed]
- Bennett S, Hurford WE. When should sedation or neuromuscular blockade be used during mechanical ventilation? Respir Care 2011;56:168-76; discussion 176-80. [Crossref] [PubMed]
- Hansen-Flaschen JH, Brazinsky S, Basile C, et al. Use of sedating drugs and neuromuscular blocking agents in patients requiring mechanical ventilation for respiratory failure. A national survey. JAMA 1991;266:2870-5. [Crossref] [PubMed]
- Reade MC, Finfer S. Sedation and delirium in the intensive care unit. N Engl J Med 2014;370:444-54. [Crossref] [PubMed]
- Smith OM, McDonald E, Zytaruk N, et al. Enhancing the informed consent process for critical care research: strategies from a thromboprophylaxis trial. Intensive Crit Care Nurs 2013;29:300-9. [Crossref] [PubMed]
- Williams BF, French JK, White HD. Informed consent during the clinical emergency of acute myocardial infarction (HERO-2 consent substudy): a prospective observational study. Lancet 2003;361:918-22. [Crossref] [PubMed]
- Salluh JI, Soares M, Teles JM, et al. Delirium epidemiology in critical care (DECCA): an international study. Crit Care 2010;14:R210. [Crossref] [PubMed]
- Pisani MA, McNicoll L, Inouye SK. Cognitive impairment in the intensive care unit. Clin Chest Med 2003;24:727-37. [Crossref] [PubMed]
- van Eijk MM, van Marum RJ, Klijn IA, et al. Comparison of delirium assessment tools in a mixed intensive care unit. Crit Care Med 2009;37:1881-5. [Crossref] [PubMed]
- Chenaud C, Merlani P, Ricou B. Informed consent for research in ICU obtained before ICU admission. Intensive Care Med 2006;32:439-44. [Crossref] [PubMed]
- Fumis RR, Ranzani OT, Martins PS, et al. Emotional disorders in pairs of patients and their family members during and after ICU stay. PLoS One 2015;10:e0115332. [Crossref] [PubMed]
- Fumis RR, Ranzani OT, Faria PP, et al. Anxiety, depression, and satisfaction in close relatives of patients in an open visiting policy intensive care unit in Brazil. J Crit Care 2015;30:440.e1-6. [Crossref] [PubMed]
- Pochard F, Azoulay E, Chevret S, et al. Symptoms of anxiety and depression in family members of intensive care unit patients: ethical hypothesis regarding decision-making capacity. Crit Care Med 2001;29:1893-7. [Crossref] [PubMed]
- Grap MJ, Munro CL. Subject recruitment in critical care nursing research: a complex task in a complex environment. Heart Lung 2003;32:162-8. [Crossref] [PubMed]
- Mehta S, Quittnat Pelletier F, Brown M, et al. Why substitute decision makers provide or decline consent for ICU research studies: a questionnaire study. Intensive Care Med 2012;38:47-54. [Crossref] [PubMed]
- Rao JK, Anderson LA, Lin FC, et al. Completion of advance directives among U.S. consumers. Am J Prev Med 2014;46:65-70. [Crossref] [PubMed]
- Gamertsfelder EM, Seaman JB, Tate J, et al. Prevalence of Advance Directives Among Older Adults Admitted to Intensive Care Units and Requiring Mechanical Ventilation. J Gerontol Nurs 2016;42:34-41. [Crossref] [PubMed]
- Silveira MJ, Kim SY, Langa KM. Advance directives and outcomes of surrogate decision making before death. N Engl J Med 2010;362:1211-8. [Crossref] [PubMed]
- Silveira MJ, Wiitala W, Piette J. Advance directive completion by elderly Americans: a decade of change. J Am Geriatr Soc 2014;62:706-10. [Crossref] [PubMed]
- Azoulay E, Pochard F, Chevret S, et al. Opinions about surrogate designation: a population survey in France. Crit Care Med 2003;31:1711-4. [Crossref] [PubMed]
- Roupie E, Santin A, Boulme R, et al. Patients' preferences concerning medical information and surrogacy: results of a prospective study in a French emergency department. Intensive Care Med 2000;26:52-6. [Crossref] [PubMed]
- Rigaud JP, Hardy JB, Meunier-Beillard N, et al. The concept of a surrogate is ill adapted to intensive care: Criteria for recognizing a reference person. J Crit Care 2016;32:89-92. [Crossref] [PubMed]
- Shalowitz DI, Garrett-Mayer E, Wendler D. The accuracy of surrogate decision makers: a systematic review. Arch Intern Med 2006;166:493-7. [Crossref] [PubMed]
- Coppolino M, Ackerson L. Do surrogate decision makers provide accurate consent for intensive care research? Chest 2001;119:603-12. [Crossref] [PubMed]
- Ciroldi M, Cariou A, Adrie C, et al. Ability of family members to predict patient's consent to critical care research. Intensive Care Med 2007;33:807-13. [Crossref] [PubMed]
- Dale C, Fowler RA, Adhikari NK, et al. Implementation of a research awareness program in the critical care unit: effects on families and clinicians. Intensive Crit Care Nurs 2010;26:69-74. [Crossref] [PubMed]
- Appelbaum PS, Roth LH, Lidz C. The therapeutic misconception: informed consent in psychiatric research. Int J Law Psychiatry 1982;5:319-29. [Crossref] [PubMed]
- Henderson GE, Churchill LR, Davis AM, et al. Clinical trials and medical care: defining the therapeutic misconception. PLoS Med 2007;4:e324. [Crossref] [PubMed]
- Lidz CW, Appelbaum PS. The therapeutic misconception: problems and solutions. Med Care 2002;40:V55-63. [Crossref] [PubMed]
- Majumdar SR, Roe MT, Peterson ED, et al. Better outcomes for patients treated at hospitals that participate in clinical trials. Arch Intern Med 2008;168:657-62. [Crossref] [PubMed]
- Janni W, Kiechle M, Sommer H, et al. Study participation improves treatment strategies and individual patient care in participating centers. Anticancer Res 2006;26:3661-7. [PubMed]
- Jansen TC, Bakker J, Kompanje EJ. Inability to obtain deferred consent due to early death in emergency research: effect on validity of clinical trial results. Intensive Care Med 2010;36:1962-5. [Crossref] [PubMed]
- Jansen TC, Kompanje EJ, Druml C, et al. Deferred consent in emergency intensive care research: what if the patient dies early? Use the data or not? Intensive Care Med 2007;33:894-900. [Crossref] [PubMed]
- Annane D, Outin H, Fisch C, et al. The effect of waiving consent on enrollment in a sepsis trial. Intensive Care Med 2004;30:321-4. [Crossref] [PubMed]
- Harvey SE, Elbourne D, Ashcroft J, et al. Informed consent in clinical trials in critical care: experience from the PAC-Man Study. Intensive Care Med 2006;32:2020-5. [Crossref] [PubMed]
- Research in emergency situations: with or without relatives consent. Emerg Med J 2004;21:703. [Crossref] [PubMed]
- Zelen M. A new design for randomized clinical trials. N Engl J Med 1979;300:1242-5. [Crossref] [PubMed]
- Harvey S, Harrison DA, Singer M, et al. Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomised controlled trial. Lancet 2005;366:472-7. [Crossref] [PubMed]
- Marshall LF, Maas AI, Marshall SB, et al. A multicenter trial on the efficacy of using tirilazad mesylate in cases of head injury. J Neurosurg 1998;89:519-25. [Crossref] [PubMed]
- Code of Federal Regulations 21CFR50.24. (Title 21, Chapter I, Subchapter A, Part 50, Section 50.24: Exception from informed consent requirements for emergency research). Available online: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=50.24
- Limkakeng AT Jr, Glickman SW, Shofer F, et al. Are patients with longer emergency department wait times less likely to consent to research? Acad Emerg Med 2012;19:396-401. [Crossref] [PubMed]
- EU clinical trials directive: 0% inspiration, 100% perspiration? Lancet Neurol 2004;3:321. [Crossref] [PubMed]
- Druml C. Informed consent of incapable (ICU) patients in Europe: existing laws and the EU Directive. Curr Opin Crit Care 2004;10:570-3. [Crossref] [PubMed]
- Kompanje EJ, Maas AI, Menon DK, et al. Medical research in emergency research in the European Union member states: tensions between theory and practice. Intensive Care Med 2014;40:496-503. [Crossref] [PubMed]
- Robinson EJ, Kerr CE, Stevens AJ, et al. Lay public's understanding of equipoise and randomisation in randomised controlled trials. Health Technol Assess 2005;9:1-192. iii-iv. [Crossref] [PubMed]
- Mentzelopoulos SD, Mantzanas M, van Belle G, et al. Evolution of European Union legislation on emergency research. Resuscitation 2015;91:84-91. [Crossref] [PubMed]
- Smart A, Thompson BT, Needham DM, et al. Surrogate consent for genetic testing, the reconsent process, and consent for long-term outcomes in acute respiratory distress syndrome trials. Am J Respir Crit Care Med 2013;188:1370-3. [Crossref] [PubMed]
- Scales DC, Smith OM, Pinto R, et al. Patients' preferences for enrolment into critical-care trials. Intensive Care Med 2009;35:1703-12. [Crossref] [PubMed]
- Dreyfuss D. To consent or not to consent, that is (not) the (sole) question. “And there is nothing new under the sun”. Kohelet (also known as Ecclesiastes), 1:9. Bible. Intensive Care Med 2004;30:180-2. [Crossref] [PubMed]
- Grunberg SM, Cefalu WT. The integral role of clinical research in clinical care. N Engl J Med 2003;348:1386-8. [Crossref] [PubMed]
- Dreyfuss D. Is it better to consent to an RCT or to care? Muetadeltaepsilonnu alphagammaalphanu ("nothing in excess"). Intensive Care Med 2005;31:345-55. [Crossref] [PubMed]
- Beecher HK. Ethics and clinical research. N Engl J Med 1966;274:1354-60. [Crossref] [PubMed]


