A few realistic questions raised by organ retrieval in the intensive care unit
Introduction
Transplantation brings sustainably-improved quality of life to patients with end-stage organ failure. In the past few decades, the need for transplants has grown more rapidly than the number of available organs. This situation of scarcity is fertile ground for illegal practices such as trade or coercive procurement of organs, transplant tourism and trafficking of human beings, practices which in turn will undermine the credibility of the legal methods.
On the other hand, many patients whose organs could potentially save the life of another, die in the intensive care unit (ICU) after a medical decision to forgo treatments deemed to be inappropriate. In some countries, most recently including France, terminally ill patients who die of circulatory arrest after a planned withdrawal of life support may be considered as organ donors under certain conditions, according to a procedure entitled “Maastricht III category”.
Since the French program started at the end of 2014, we examine some of the practical, legal and ethical issues that arise when considering organ donation in the context of end-of-life decisions. We thus address factors determining how life-sustaining treatment is to be withdrawn, debates relating to the diagnosis and time of death, and identification of the donor’s overall benefit.
A shortage of organs is fertile ground for illegal practices
Organ transplantation increases life expectancy and offers a better quality of life with the best cost-benefit ratio as compared to other organ-substitution therapies. It increases the opportunities for patients to participate in social, working and sporting activities. In the past few decades, the need for transplants has grown faster than the number of available organs. Qualified as a worldwide shortage, the widening gap between organ demand (i.e., patients in terminal organ failure) and donor graft supply is forcing a rethink of the practical and ethical issues surrounding organ transplantation. French policy on organ retrieval essentially hinges on brain dead donors (termed “heart-beating donors”). Over the past decade, organ donation following traumatic brain death has become scarcer. Efforts to maintain a pool of available grafts revolved around extending the donor selection criteria to include elderly and/or chronically ill patients (such as diabetics or subjects with arterial hypertension) whose death mostly results from cerebrovascular accidents. This policy seems to have reached its limits, and can no longer meet the demand for transplants.
The shortage of available organs in the legal system encourages illegal practices that in turn could undermine the credibility of the conventional methods (living and deceased donation). These illegal practices include organ sales, coercive procurement of organs, transplant tourism and trafficking of human beings for the purpose of organ retrieval. Patients in need of organs with sufficient resources may travel in economically emerging countries to purchase a kidney mainly from poor individuals (1). It is estimated that organ trafficking accounts for 5–10% of the kidney transplants performed annually throughout the world (1). In the context of a global shortage of organs, the World Health Organization (WHO) called on member states in 2004 to address the urgent problems of organ sales, transplant tourism and trafficking in organ donors, and to take measures to protect vulnerable groups from such practices. With the adoption of the Istanbul declaration in May 2008 by more than 150 representatives of scientific and medical bodies from around the world, government officials, social scientists, and ethicists, trading and trafficking organs should definitively be prohibited in (and from) all signatory countries (2).
Patients whose organs could potentially save lives are dying every day in ICUs
Some countries have developed all or part of their transplantation policy on donation after circulatory determination of death (CDD), so-called “non-heart-beating donation” (3-6). In 1995, Dutch transplant surgeons distinguished four circumstances of CDD into what is known as the Maastricht classification (7): unforeseeable irreversible circulatory arrest without (category I) or with (category II) immediate cardiopulmonary resuscitation attempted by trained providers (uncontrolled CDD), foreseeable circulatory arrest occurring after a decision to forego life-sustaining treatment (category III, controlled CDD), circulatory arrest occurring after brain death (category IV). Donations after unforeseeable irreversible circulatory arrest (uncontrolled CDD, left-hand panel of Figure 1) are authorized in France since 2005 (9). As the procedure is restricted to a small number of suitably-equipped centers, relatively few organs have been retrieved under this system. A persisting shortfall in available organs prompted French authorities and practitioners to focus on organ retrieval in patients withdrawn from life-sustaining treatment and awaiting circulatory arrest (controlled CDD, Maastricht classification category III).
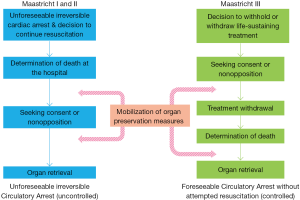
Terminally ill patients who die of circulatory arrest after a planned withdrawal of life support may be considered as organ donors under certain conditions. Prior to 2005, French regulations were not designed for such practices. With regard to patients in the final stages of incurable disease, law number 2005-370 dated April 22, 2005 authorizes the withholding or withdrawal (WhWd) of treatments when they appear “useless, disproportionate or having no other effect than solely the artificial preservation of life”. Advocates of organ donation after controlled CDD (right-hand panel of Figure 1) argue that the end-of-life care plan should incorporate the patient’s desires concerning organ donation and the public interest of transplantation. In many countries, teams involved in organ procurement after death (whatever the circumstances) consider organ and tissue retrieval as a routine part of end-of-life care, once it is established that the patient wishes to be a donor (10-13). However, until 2013, most French academics regarded the perceived conflict of interest that would arise for clinicians treating potential donors as a major ethical question, emphasizing that such procedures could be experienced as a form of utilitarian end-of-life practice (10,14-16). In 2013, a regulatory framework making this type of organ donation possible was debated in the French parliament. A dedicated steering committee drafted a protocol establishing the mandatory conditions to retrieve organs under the Maastricht III setting in France (17). The program officially got underway in December 2014.
Caregivers have equal responsibility towards both the dying patient and the patient awaiting transplant
There is significant variation in how treatment withdrawals are implemented in ICUs, particularly with regard to airway management (18). Published guidelines mainly focus on the decision-making principles rather than practical details about how end-of-life care should be managed (19,20). Once artificial breathing support is switched off, it becomes possible to remove the endotracheal tube that connects the patient to the ventilator and secures the airway (10). Rather than an abrupt “on-off” discontinuation of mechanical ventilation, with or without extubation (removal of the endotracheal tube), many teams prefer a progressive withdrawal of mechanical ventilation (termed “terminal weaning”), as they feel the physical symptoms of airway obstruction may harm the patient and be distressing to relatives and caregivers (21). However, some consider this progressive weaning as an unnecessarily prolonged agony if death is the only possible outcome (22), especially since these distressing symptoms might be thoroughly anticipated (23). In either case, once a life-support withdrawal decision has been made, delivering comfort care becomes priority. While the technical environment of ICUs does not offer optimal conditions for a quiet end-of-life, therapies from this point mainly focus on relieving pain, anxiety and discomfort.
Enrolling death into an organ retrieval procedure entails a number of organizational constraints that may interfere with the comfort care traditionally given to dying patients and their loved ones. Under Maastricht III conditions, in order to meet the time framework tied to organ viability, life-support is withdrawn either in the operating room or in the ICU, provided that the patient can be swiftly transferred to the operating room once death is certified (12). These operational requirements contrast with the regular palliative approach (i.e., with no intention of organ retrieval). Even though it is theoretically possible to maintain contact between the patient and relatives up to surgical intervention, the technical environment of an operating room is far from the ideal place to organize spiritual assistance and end-of-life rituals (24). Furthermore, the quality of the organs harvested under such conditions is closely dependent on how early technical organ preservation measures are implemented. One of these technical measures consists in catheterizing the aorta and inferior vena cava in order to connect an extracorporeal pump and maintain circulation in the abdominal organs. Once life-sustaining treatments get discontinued according to a formal collegial debate, any intrusive intervention practiced before the subject is declared dead could be seen as conflicting with efforts to deliver terminal supportive care. Thus, a formal policy regarding the comfort of both patients and relatives throughout the procedure is essential for the acceptance of organ donation under Maastricht III conditions. Since caregivers represent the interests of both the potential donor and the persons awaiting a transplant (an approach called “dual advocacy”), high-level palliative care would converge with organ transplantation so as to transform a respectful death into a promise of life for others in need (25).
Withdrawal of life support for highly-dependent patients is the only situation compatible with organ donation
The period between withdrawal of life-sustaining treatment and death (the so-called “withdrawal period”) is a major determinant of organ donation and of the quality of the organs retrieved for transplantation (26,27). It is not the duration per se but rather the hemodynamic profile during the withdrawal period that determines the consequences of warm ischemia on organ viability (26). However, a long withdrawal period often results in severe ischemic damage, compromising organ usability for transplantation (26,28). This period may range from a few minutes to many hours or days, depending on the level of life support engaged at the time of the decision for WhWd, and how withdrawal is achieved. Because circulatory arrest must occur after a short period, only the withdrawal of life-sustaining cardiopulmonary support for highly-dependent patients [high inspired oxygen fraction (FiO2), non-triggered modes of ventilation, inotrope/vasoactive drug use] is compatible with post-mortem organ donation (26,28-32). Any patient in whom the elective WhWd measure is not withdrawal of life-sustaining treatment should therefore be definitively excluded from any intention to retrieve organs.
Such a procedure could even become intolerable for relatives and caregiving staff if the eventuality (still possible) of a prolonged agonal period making organ donation impossible has not been explicitly addressed beforehand. It is thus essential to accurately predict time to circulatory arrest after withdrawal of life-sustaining treatment (28,29,31,33,34). When death is the most likely outcome, withdrawal of life-sustaining treatments usually involves disconnection of mechanical ventilation (with or without removal of the endotracheal tube) and cessation of vasoactive drugs. Removal of the endotracheal tube (extubation) is more often associated with progression to organ donation than terminal weaning without extubation (12). Death within one or two hour(s) of withdrawal usually correlates with severe brain injuries (low Glasgow Coma Scale, absence of brainstem reflexes) (30-32,35-38), high dependence on mechanical ventilation (non-triggered mode, high FiO2, high positive expiratory pressure) (28-32,36,38,39), use of inotrope drugs (29,30,35,39), young age (28,35,40), underlying diseases (37,39), and physiological anomalies (high severity index scores, low blood pressure, low pH on arterial blood gas analysis) (37,38,40,41). Under Maastricht III conditions, the removal of organs must be scheduled before withholding/withdrawal implementation and starts as soon as death is certified. As removal of organs should not precede the donor’s death (so as to fulfill the “dead donor rule”), defining the precise moment of death after withdrawal requires very explicit criteria to be determined, despite the lack of biological evidence supporting this accuracy (42,43). Several organizations state that “if the patient or surrogate understands the circumstances of the determination of death”, physicians are legally authorized to declare death after 2 minutes of absent circulation (44).
Brain-injured patients are more likely to die under circumstances which may fulfill the Maastricht III conditions
Severely brain-injured patients are more likely to die after withdrawal of life-sustaining treatments, in circumstances which may fulfill the requirements for organ retrieval under Maastricht III conditions (8,12,32). Contrary to ethicists (22,45,46), many intensivists clearly distinguish between “withholding” and “withdrawal” decisions, with the former being perceived as more passive (47-52). By establishing a three-level hierarchy of decisions (“stop” > “do not increase” > “do not start”), a French epidemiological survey demonstrated that the more “active” limitations (withdrawal of life-sustaining therapy) mostly involved severely brain-injured patients (post-anoxic coma, stroke, head trauma), whereas patients with chronic respiratory disease, pre-existing disability affecting autonomy or cognition, and/or respiratory failure on admission had treatment preferentially withheld rather than withdrawn (53). This study was conducted before the Maastricht III program was launched in France, under conditions enabling a state-of-play of practices without the physicians responsible for WhWd decisions being pressured by any ethical dilemma between the obligation to accompany the dying patient and the need to retrieve the patient’s organs. One potential explanation is that prognostic indices based on several factors in combination may predict outcome with better accuracy in neuro-critical care than in other areas in medicine (54-58). For patients with congestive heart failure, obstructive bronchitis, cirrhosis, kidney disease or cancer, it is rarely possible to prognosticate with certainty that a chronically ill subject will not survive an acute episode (59). However, at an individual level, available prognostic indices are not accurate enough to make definite end-of-life decisions without foretelling a destiny that would become self-fulfilling (“self-fulfilling prophecy”) (60-65). In addition, most prediction models were not developed with the specific aim of informing end-of-life decisions (58,62).
In case of brain injury, the predicted outcome measure is either death or poor functional fate. Continuation of treatment may prolong life for months or years at the cost of being in a severely disabled state that these patients would not have accepted (58). Yet, “against all odds”, many people with serious and persistent disabilities report afterwards a good quality of life, although most external observers (physicians and relatives) consider that they live an undesirable existence. This phenomenon is known as “the disability paradox” (66). Brain-injured patients are rarely or never conscious at the time of the decision-making and cannot be involved in the discussions. In the survey mentioned above, the low level of patients being directly or indirectly involved in the decision-making (23%) may reflect that many were unable to express their preferences once hospitalized, and/or that they did not anticipate such conditions of being before admission (53). While the French law authorizing such practices was passed in 2005, the prevalence of advance directives and designated surrogate persons remains low (53). When patients in the ICU lack decision-making capacity, WhWd discussions are often shared between physicians, nurses, and family members or relatives acting as surrogates and representing the patient’s values and preferences (67,68). Once a WhWd decision has been made, a physician who has no direct knowledge of the deceased’s wishes must question the relatives about a possible consent/opposition to organ or tissue donation expressed during the patient’s lifetime. However, because the patient’s wishes are rarely known at the time of the deliberation, decisions concerning WhWd (and organ donation) remain primarily based on medical authority and substituted judgment (69,70).
Everybody should be offered “a right to donate” whatever the circumstances of death
Brain-injured patients are more likely to die under circumstances which may fulfill the technical requirements for a Maastricht III procedure, whereas they are rarely or never conscious at the time of decision-making, and empirically have the poorest ability to participate in the discussion. Practice irrespective of the rule (i.e., first-person consent) would consist in determining whether close relatives under emotional stress wish to donate their loved one’s organs. Many of those who deny donation regret their decision soon after the funeral (71). If one considers the interest of potential recipients to be pre-emptive over all other considerations, a majority of the community expresses the belief that cadaver organs should be used for transplantation. An adequate regulatory framework regarding the use of organs for transplantation and the implementation of high-level quality and safety criteria fortify the trust of all relevant stakeholders (citizens, donors, recipients and caregivers) in the area of transplantation. Competent authorities should also consider correcting people’s false assumptions and taboos in this area, and encouraging discussion about the therapeutic usability of organs for the living. Public awareness of the possibility to donate one’s organs and that those organs are allocated to recipients free of charge, according to transparent and non-discriminatory criteria should be enhanced (72). Every citizen should be offered a “right to donate” whatever the circumstances of their death (death after circulatory arrest, brain death), whenever and wherever it occurs, and thus be ensured that their wishes will be respected after death (73). Individuals should have opportunity to enroll in a national organ/tissue-donor register when completing certain administrative formalities such as applying for a passport, a driving licence or a health insurance card (72). The European parliament resolution of 19 May 2010 urges the EU states to look into adopting a program of on-line enrolment in a national or international donor register (72).
One possible solution (cited above and already established in certain countries) consists in registering an explicit consent/opposition to organ donation, modifiable at all times, recorded in a national computerised registry. However, regardless of the system of consent that is in place (opt in or opt out), individuals should above all be aware of the legislation in force. Guibet Lafaye and Kreis proposed that the health insurance card (or other personal document) could act as a support, mentioning not whether an individual agrees to donate his/her organs after death (a decision that remains revocable at all times), but rather, whether he/she is fully aware of the legislation in force (74). This registration would be coupled with a public obligation to inform (general practitioners, schools, universities, military draft, public administrations…). The recording (or absence thereof) in the consent/opposition registry, combined with the mention of the individual’s awareness of the legislation in force, should ensure that the individual’s wishes will be respected after death, thus relieving the deceased’s surrogates of the emotional burden of decision-making (74). Families and organ procurement coordinators would no longer have to confront the emotional, and laborious question of the patient’s preferences. Time spent with relatives could be preferentially and usefully employed on other important issues such as the vital benefit for the recipient and the guarantee of protection from “desecration” for the deceased. Furthermore, participation of families in decisions that respect their loved one’s wishes could help to ease the grieving process. By asking citizens to make a choice or at least to be aware of the legislation in force, the state would encourage a responsible exercise of autonomy while minimizing intrusion into individual autonomy (75).
Conclusions
Every citizen wishing to donate organs or tissues after death should be assured that their willingness will be respected once deceased or severely disabled. Unfortunately, the rights to govern one’s own health conferred on citizens by law (advance directives, designated surrogates, national registers) appear to be under-used. Because organs cannot be appropriated against the living individual’s will, a personal document prepared beforehand should mention whether an individual wishes to donate, or at least whether he/she is aware of the legislation regarding transplantation. Relatives and caregivers would then no longer have to challenge the laborious question of the patient’s preferences.
Based on the concept of “dual advocacy” that simultaneously takes into account the interests of the dying patient and those of potential recipients, end-of-life palliative care and organ donation are not incompatible, once it is established that the patient wished to be a donor. In such situations, caregivers must tackle the care for the dying (and relatives) and the purpose of organ retrieval with equal determination. However, it seems crucial to focus on the factors determining how and when life support has to be withdrawn in the ICU, particularly discontinuing mechanical ventilation and removing the endotracheal tube, with a sensitive issue unavoidably arising: in which conditions are we medically and ethically authorized to revise our practices and make them suitable for organ donation after circulatory death?
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Budiani-Saberi DA, Delmonico FL. Organ trafficking and transplant tourism: a commentary on the global realities. Am J Transplant 2008;8:925-9. [Crossref] [PubMed]
- The declaration of Istanbul on organ trafficking and transplant tourism. Nephrol Dial Transplant 2008;23:3375-80. [Crossref] [PubMed]
- DeVita MA, Snyder JV. Development of the University of Pittsburgh Medical Center policy for the care of terminally ill patients who may become organ donors after death following the removal of life support. Kennedy Inst Ethics J 1993;3:131-43. [Crossref] [PubMed]
- Shemie SD, Baker AJ, Knoll G, et al. National recommendations for donation after cardiocirculatory death in Canada: Donation after cardiocirculatory death in Canada. CMAJ 2006;175:S1. [Crossref] [PubMed]
- Reich DJ, Mulligan DC, Abt PL, et al. ASTS recommended practice guidelines for controlled donation after cardiac death organ procurement and transplantation. Am J Transplant 2009;9:2004-11. [Crossref] [PubMed]
- Ysebaert D, Van Beeumen G, De Greef K, et al. Organ procurement after euthanasia: Belgian experience. Transplant Proc 2009;41:585-6. [Crossref] [PubMed]
- Kootstra G, Daemen J, Oomen A, et al. Categories of non-heart-beating donors. Transplant Proc 1995;27:2893-4. [PubMed]
- Lesieur O, Mamzer MF, Leloup M, et al. Eligibility of patients withheld or withdrawn from life-sustaining treatment to organ donation after circulatory arrest death: epidemiological feasibility study in a French Intensive Care Unit. Ann Intensive Care 2013;3:36. [Crossref] [PubMed]
- Décret n° 2005-949 du 2 août 2005 relatif aux conditions de prélèvement des organes, des tissus et des cellules et modifiant le livre II de la première partie du code de la santé publique. Journal Officiel de la République Française du 6 aout 2005 [Internet]. Available online: http://www.legifrance.gouv.fr
- Graftieaux JP, Bollaert PE, Haddad L, et al. Contribution of the ethics committee of the French Intensive Care Society to describing a scenario for implementing organ donation after Maastricht type III cardiocirculatory death in France. Ann Intensive Care 2012;2:23. [Crossref] [PubMed]
- Price D. End-of-life treatment of potential organ donors: paradigm shifts in intensive and emergency care. Med Law Rev 2011;19:86-116. [Crossref] [PubMed]
- Manara AR, Murphy PG, O’Callaghan G. Donation after circulatory death. Br J Anaesth 2012;108 Suppl 1:i108-21. [Crossref] [PubMed]
- Coggon J. Elective ventilation for organ donation: law, policy and public ethics. J Med Ethics 2013;39:130-4. [Crossref] [PubMed]
- Cabrol C. Organ procurement from non-heart-beating donors. Bull Académie Natl Médecine 2007;191:633-8.
- OPINION n° 115. Ethical issues in connection with organ harvesting and donation for transplanting. Available online: http://www.ccne-ethique.fr/sites/default/files/publications/avis_115eng_0.pdf
- Puybasset L, Bazin JE, Beloucif S, et al. Critical appraisal of organ procurement under Maastricht 3 condition. Ann Fr Anesth Reanim 2014;33:120-7. [Crossref] [PubMed]
- Antoine C, Mourey F, Prada-Bordenave E, et al. How France launched its donation after cardiac death program. Ann Fr Anesth Reanim 2014;33:138-43. [Crossref] [PubMed]
- Cottereau A, Robert R, le Gouge A, et al. ICU physicians’ and nurses’ perceptions of terminal extubation and terminal weaning: a self-questionnaire study. Intensive Care Med 2016;42:1248-57. [Crossref] [PubMed]
- Myburgh J, Abillama F, Chiumello D, et al. End-of-life care in the intensive care unit: Report from the Task Force of World Federation of Societies of Intensive and Critical Care Medicine. J Crit Care 2016;34:125-30. [Crossref] [PubMed]
- Downar J, Delaney JW, Hawryluck L, et al. Guidelines for the withdrawal of life-sustaining measures. Intensive Care Med 2016;42:1003-17. [Crossref] [PubMed]
- Rady MY, Verheijde JL. The science and ethics of withdrawing mechanical positive pressure ventilatory support in the terminally ill. J Palliat Med 2013;16:828-30. [Crossref] [PubMed]
- Wilkinson D, Savulescu J. A costly separation between withdrawing and withholding treatment in intensive care. Bioethics 2014;28:127-37. [Crossref] [PubMed]
- Kompanje EJ, van der Hoven B, Bakker J. Anticipation of distress after discontinuation of mechanical ventilation in the ICU at the end of life. Intensive Care Med 2008;34:1593-9. [Crossref] [PubMed]
- Le Breton D. Aspects anthropologiques et culturels du corps. In: Ethique et transplantations d’organes, Jean François Collange. Paris: Ellipses, 2000:39-49.
- Lazaridis C. Transforming ICU death into life-radically more. Intensive Care Med 2016;42:2096-7. [Crossref] [PubMed]
- Bradley JA, Pettigrew GJ, Watson CJ. Time to death after withdrawal of treatment in donation after circulatory death (DCD) donors. Curr Opin Organ Transplant 2013;18:133-9. [Crossref] [PubMed]
- Scalea JR, Redfield RR, Arpali E, et al. Does DCD donor Time-to-Death affect recipient outcomes? implications of Time-to-Death at a High-Volume center in the United States. Am J Transplant 2017;17:191-200. [Crossref] [PubMed]
- Suntharalingam C, Sharples L, Dudley C, et al. Time to cardiac death after withdrawal of life-sustaining treatment in potential organ donors. Am J Transplant 2009;9:2157-65. [Crossref] [PubMed]
- Wind J, Snoeijs MG, Brugman CA, et al. Prediction of time of death after withdrawal of life-sustaining treatment in potential donors after cardiac death*. Crit Care Med 2012;40:766-9. [Crossref] [PubMed]
- Devita MA, Brooks MM, Zawistowski C, et al. Donors after cardiac death: validation of identification criteria (DVIC) study for predictors of rapid death. Am J Transplant 2008;8:432-41. [Crossref] [PubMed]
- Rabinstein AA, Yee AH, Mandrekar J, et al. Prediction of potential for organ donation after cardiac death in patients in neurocritical state: a prospective observational study. Lancet Neurol 2012;11:414-9. [Crossref] [PubMed]
- Lesieur O, Leloup M, Gonzalez F, et al. Eligibility for organ donation following end-of-life decisions: a study performed in 43 French intensive care units. Intensive Care Med 2014;40:1323-31. [Crossref] [PubMed]
- Munshi L, Dhanani S, Shemie SD, et al. Predicting time to death after withdrawal of life-sustaining therapy. Intensive Care Med 2015;41:1014-28. [Crossref] [PubMed]
- He X, Xu G, Liang W, et al. Nomogram for predicting time to death after withdrawal of Life-Sustaining treatment in patients with devastating neurological injury. Am J Transplant 2015;15:2136-42. [Crossref] [PubMed]
- Davila D, Ciria R, Jassem W, et al. Prediction models of donor arrest and graft utilization in liver transplantation from maastricht-3 donors after circulatory death. Am J Transplant 2012;12:3414-24. [Crossref] [PubMed]
- De Groot YJ, Lingsma HF, Bakker J, et al. External validation of a prognostic model predicting time of death after withdrawal of Life support in neurocritical patients. Crit Care Med 2012;40:233-8. [Crossref] [PubMed]
- Epker JL, Bakker J, Kompanje EJ. The use of opioids and sedatives and time until death after withdrawing mechanical ventilation and vasoactive drugs in a Dutch intensive care unit. Anesth Analg 2011;112:628-34. [Crossref] [PubMed]
- Brieva J, Coleman N, Lacey J, et al. Prediction of death in less than 60 minutes following withdrawal of cardiorespiratory support in ICUs. Crit Care Med 2013;41:2677-87. [Crossref] [PubMed]
- Lewis J, Peltier J, Nelson H, et al. Development of the University of Wisconsin donation after Cardiac death evaluation tool. Prog Transplant 2003;13:265-73. [Crossref] [PubMed]
- Cooke CR, Hotchkin DL, Engelberg RA, et al. Predictors of time to death after terminal withdrawal of mechanical ventilation in the ICU. Chest 2010;138:289-97. [Crossref] [PubMed]
- Pine JK, Goldsmith PJ, Ridgway DM, et al. Predicting donor asystole following withdrawal of treatment in donation after cardiac death. Transplant Proc 2010;42:3949-50. [Crossref] [PubMed]
- Shemie SD, Hornby L, Baker A, et al. International guideline development for the determination of death. Intensive Care Med 2014;40:788-97. [Crossref] [PubMed]
- Truog R. The price of our illusions and myths about the dead donor rule. J Med Ethics 2016;42:318-9. [Crossref] [PubMed]
- Gries CJ, White DB, Truog RD, et al. An official American Thoracic Society/International Society for Heart and Lung Transplantation/Society of Critical Care Medicine/Association of Organ and Procurement Organizations/United Network of Organ Sharing Statement: ethical and policy considerations in organ donation after circulatory determination of death. Am J Respir Crit Care Med 2013;188:103-9. [Crossref] [PubMed]
- Curtis JR, Vincent JL. Ethics and end-of-life care for adults in the intensive care unit. Lancet 2010;376:1347-53. [Crossref] [PubMed]
- Truog RD, Campbell ML, Curtis JR, et al. Recommendations for end-of-life care in the intensive care unit: a consensus statement by the American College [corrected] of Critical Care Medicine. Crit Care Med 2008;36:953-63. [Crossref] [PubMed]
- Vincent JL. Forgoing Life support in western European intensive care units: the results of an ethical questionnaire. Crit Care Med 1999;27:1626-33. [Crossref] [PubMed]
- Sprung CL, Cohen SL, Sjokvist P, et al. End-of-life practices in European intensive care units: the Ethicus Study. JAMA 2003;290:790-7. [Crossref] [PubMed]
- Azoulay E, Metnitz B, Sprung CL, et al. End-of-life practices in 282 intensive care units: data from the SAPS 3 database. Intensive Care Med 2009;35:623-30. [Crossref] [PubMed]
- Phua J, Joynt GM, Nishimura M, et al. Withholding and withdrawal of life-sustaining treatments in intensive care units in Asia. JAMA Intern Med 2015;175:363-71. [Crossref] [PubMed]
- Metaxa V, Lavrentieva A. End-of-life decisions in Burn Intensive Care Units - An International Survey. Burns 2015;41:53-7. [Crossref] [PubMed]
- Chung GS, Yoon JD, Rasinski KA, et al. US Physicians' Opinions about Distinctions between Withdrawing and Withholding Life-Sustaining Treatment. J Relig Health 2016;55:1596-606. [Crossref] [PubMed]
- Lesieur O, Leloup M, Gonzalez F, et al. Withholding or withdrawal of treatment under French rules: a study performed in 43 intensive care units. Ann Intensive Care 2015;5:56. [Crossref] [PubMed]
- Verkade MA, Epker JL, Nieuwenhoff MD, et al. Withdrawal of life-sustaining treatment in a mixed intensive care unit: most common in patients with catastropic brain injury. Neurocrit Care 2012;16:130-5. [Crossref] [PubMed]
- Côte N, Turgeon AF, Lauzier F, et al. Factors associated with the withdrawal of life-sustaining therapies in patients with severe traumatic brain injury: a multicenter cohort study. Neurocrit Care 2013;18:154-60. [Crossref] [PubMed]
- Kamps MJ, Horn J, Oddo M, et al. Prognostication of neurologic outcome in cardiac arrest patients after mild therapeutic hypothermia: a meta-analysis of the current literature. Intensive Care Med 2013;39:1671-82. [Crossref] [PubMed]
- Parry-Jones AR, Abid KA, Di Napoli M, et al. Accuracy and clinical usefulness of intracerebral hemorrhage grading scores: a direct comparison in a UK population. Stroke 2013;44:1840-5. [Crossref] [PubMed]
- Geurts M, Macleod MR, Van Thiel GJ, et al. End-of-life decisions in patients with severe acute brain injury. Lancet Neurol 2014;13:515-24. [Crossref] [PubMed]
- White DB, Ernecoff N, Billings JA, et al. Is dying in an ICU a sign of poor quality end-of-life care? Am J Crit Care 2013;22:263-6. [Crossref] [PubMed]
- Zamperetti N, Piccinni P. Intensivists managing end-of-life care: dwarfs without giants' shoulders to stand upon. Intensive Care Med 2010;36:1985-7. [Crossref] [PubMed]
- Frost DW, Cook DJ, Heyland DK, et al. Patient and healthcare professional factors influencing end-of-life decision-making during critical illness: a systematic review. Crit Care Med 2011;39:1174-89. [Crossref] [PubMed]
- Christakis N. Death foretold: prophecy and prognosis in medical care. Chicago: University of Chicago Press, 2001.
- Ong CJ, Dhand A, Diringer MN. Early withdrawal decision-making in patients with coma after cardiac arrest: a qualitative study of intensive care clinicians. Neurocrit Care 2016;25:258-65. [Crossref] [PubMed]
- Zahuranec DB, Fagerlin A, Sanchez BN, et al. Variability in physician prognosis and recommendations after intracerebral hemorrhage. Neurology 2016;86:1864-71. [Crossref] [PubMed]
- Hwang DY, Dell CA, Sparks MJ, et al. Clinician judgment vs formal scales for predicting intracerebral hemorrhage outcomes. Neurology 2016;86:126-33. [Crossref] [PubMed]
- Albrecht GL, Devlieger PJ. The disability paradox: high quality of Life against all odds. Soc Sci Med 1999;48:977-88. [Crossref] [PubMed]
- Kon AA, Davidson JE, Morrison W, et al. Shared decision making in ICUs: an American college of critical care medicine and American thoracic society policy statement. Crit Care Med 2016;44:188-201. [Crossref] [PubMed]
- Cai X, Robinson J, Muehlschlegel S, et al. Patient preferences and surrogate decision making in neuroscience intensive care units. Neurocrit Care 2015;23:131-41. [Crossref] [PubMed]
- Torke AM, Alexander GC, Lantos J. Substituted judgment: the limitations of autonomy in surrogate decision making. J Gen Intern Med 2008;23:1514-7. [Crossref] [PubMed]
- de Groot J, van Hoek M, Hoedemaekers C, et al. Decision making on organ donation: the dilemmas of relatives of potential brain dead donors. BMC Med Ethics 2015;16:64. [Crossref] [PubMed]
- Morais M, Da Silva RC, Duca WJ, et al. Families who previously refused organ donation would agree to donate in a new situation: a cross-sectional study. Transplant Proc 2012;44:2268-71. [Crossref] [PubMed]
- European Parliament resolution of 19 May 2010 on the Commission Communication: Action plan on Organ Donation and Transplantation (2009-2015): Strengthened Cooperation between Member States. Available online: http://www.europarl.europa.eu/sides/getDoc.do?type=TA&reference=P7-TA-2010-0183&language=EN&ring=A7-2010-0103
- Glannon W. Do the sick have a right to cadaveric organs? J Med Ethics 2003;29:153-6. [Crossref] [PubMed]
- Guibet Lafaye C, Kreis H. From altruistic donation to conditional societal organ appropriation after death. Ethical Theory Moral Pract 2013;16:355-68. [Crossref]
- Giordano S. Is the body a republic? J Med Ethics 2005;31:470-5. [Crossref] [PubMed]


