Cystic adventitial disease—case series and review of literature
Case presentation
Case 1
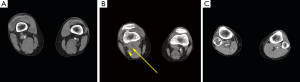
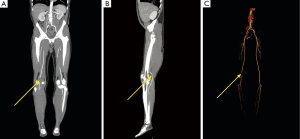
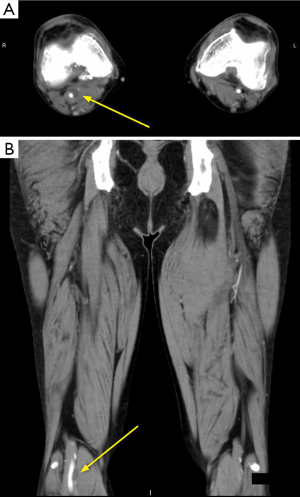
A 54-year-old male who presented after an abnormal ultrasound (US) with Doppler exam at an outside institution with a 2 months history of new, progressive right leg claudication. His medical history is significant for hypertension and a maternal history of fatal abdominal aortic aneurysm rupture. The patient states a history of trauma to the right knee of uncertain nature. He denies illicit drug use but confirms a long history of cigarette smoking. Review of systems is otherwise negative. On physical examination, no swelling, varicose veins or stiffness in the right leg were identified. The right pedal pulses were palpable but greatly reduced. The left leg is unremarkable. The initial abnormal Doppler US performed at an outside institution (image not available) reported a 1.5 cm right popliteal artery aneurysm with a possible dissection within this aneurysm and luminal narrowing. An urgent computed tomography angiography (CTA) abdomen and pelvis with runoff was performed which demonstrated a cystic structure intimately associated with the right above-the-knee popliteal artery, causing near complete occlusion (Figures 1,2) without evidence of dissection. A presumptive diagnosis of cystic adventitial disease (CAD) is made at this time, and the patient was taken to the operating room for definitive diagnosis and management. A posterior surgical approach was taken and a cyst in the wall of the right popliteal artery was identified. The cyst was entered with the evacuation of viscous, jelly-like amber material. The entire cyst wall was resected. There was strong distal popliteal artery pulse status post cystectomy. The patient had an unremarkable recovery. Follow up with Doppler US performed at 2 and 4 months post-cystectomy showed a normal right popliteal artery, without evidence of cyst recurrence.
Case 2
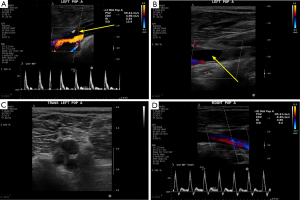
A 58-year-old male developed an acute onset of left lower extremity claudication and numbness. There were no resting pain, skin ulcerations or prior history of claudication symptoms. On physical examination, the patient’s right lower extremity pulses were within normal limits. The left lower extremity pedal pulses were palpable but reduced, and became nonpalpable with left knee flexion (positive Ishikawa sign). A Doppler US demonstrated an anechoic lesion intimately associated with the left popliteal artery causing significant narrowing (Figure 3). Subsequent CTA abdomen and pelvis with runoff showed a hypodense lesion causing irregular narrowing of the left popliteal artery (Figure 4). This lesion did not show flow on Doppler or contrast opacification on CTA runoff. Differential diagnosis at this time includes CAD and peripheral embolism. Intraoperatively, a cyst within the walls of the left popliteal artery was found. Viscous fluid was aspirated from the cyst, followed by complete resection of the cyst. The patient had some incisional soreness but otherwise had an unremarkable recovery. Follow up at 6 weeks and 6 months post-cystectomy with Doppler US did not show any stenosis of the left popliteal artery nor any recurring cyst.
Case 3
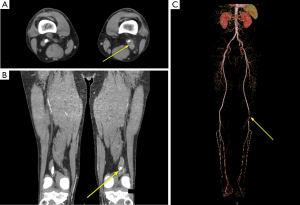
A 55-year-old male who was referred after developing progressively worsening right calf pain for 3 to 4 weeks, not associated with any trauma or other notable inciting event. Right calf pain was associated with cramping and burning sensation. There were three unknown surgical procedures performed on the right knee previously. Prior to presenting, a US done at an outside institution for deep vein thrombosis displayed an anechoic, avascular structure adjacent to the right popliteal artery (image not available). CTA runoff showed a hypodensity adjacent the right popliteal artery at the knee with moderate compression and stenosis of the popliteal artery lumen (Figure 5). Intraoperatively, a cyst contained viscous fluid was identified in the wall of the right popliteal artery. Complete cystectomy was performed after evacuation of the cyst contents. Follow up US at 6 weeks, 6 months and then annually for 2 years were negative for recurrence of the right popliteal artery cyst.
Discussion
Etiology and pathophysiology
CAD is a rare vascular disorder, first described by Atkins and Key in 1947 as a case of myxomatous tumor in the left external iliac artery (1). Since that initial description, approximately 350 cases has been reported in the literature (2).
A number of theories have been proposed for the cause of CAD. The earliest proposed theory by Linquette in 1967 suggested that the de novo mucinous degeneration in the setting of CAD is associated with a generalized/systemic disorder without much substantiating evidence (3).
A second theory focus on repeated trauma, based on the fact that most CAD occur in the popliteal artery (4). Theoretically, the popliteal artery is subjected to repetitive stretching and distortion, causing destruction as well as cystic degeneration of the vessel adventitia. The repeated shearing force cause small detachments of the adventitia from the media with intramural hemorrhage which then subsequently develop into the cysts within the adventitial (5,6). Although the trauma theory enjoys the most support, there are also flaws. In most cases of CAD, there is a lack of history of trauma. CAD has also been reported to occur in younger patients, presumably prior to exposure to excessive trauma. Furthermore, the trauma theory does not account for CAD in deep vessels, or the lack of increased incidence in subpopulations with increased risk of trauma, such as athletes (7).
A modified theory based on repeated trauma is proposed as the articular or synovial theory; it differs from the trauma theory by propose that trauma to the adjacent joints is the underlying cause of CAD (5,8-10). This theory suggests that repeated trauma to the joints cause damage to the joint capsule, result in tracking of the synovial fluid to the adjacent arterial vasculature which can subsequently lead to development of the cysts. Desy et al. substantiated this theory by identifying a number of cases where an interconnection was seen between the synovium and the cysts, as well as by the connection between different geniculate artery cysts (11). CAD in vessels that are not in immediate neighborhood of a joint is explained by Desy et al. as caused by migration of synovial cells along the arterial vessel.
The fourth theory of CAD origin is the developmental theory. This theory posits that mucin secreting mesenchymal cells are placed in the adventitia of the vessels during embryogenesis. These mesenchymal cells secretes mucin later on in life, which enlarge to form the cysts (5,7). A variation of this theory is that the vessel incorporated an adjacent ganglion cyst during development, due to the macroscopic similarities of cysts in CAD and ganglion cysts (12). However, the ganglion theory does not enjoy much support due to the wide differences in histochemistry between ganglion and CAD cysts.
Currently, no single theory is universally accepted as the pathogenesis of CAD, with the ganglion/articular theory and developmental theory enjoys the most support. Supporter of each theory explain common findings differently; as an example, image finding of communication between the synovial joint and the adventitial cysts is explained as the reason for cyst formation due to trauma by the ganglion/articular theorists, whilst it is seen as the embryologic remnant by the developmental theorists. We do not have any inclination to a particular theory, but feel there should be further investigation and analysis.
Epidemiology and anatomic distribution
CAD predominantly affects the arteries, although rare reports of CAD of the veins have also been described. Majority (85%) of the cysts in CAD are found in the popliteal artery but have also been reported in the external iliac, femoral, radial, ulnar, brachial, and axillary arteries. A rare case involving the abdominal aorta has also been reported (13,14). CAD predominantly occur in the young to middle-aged population; although the age of presentation ranges from 11 to 70 years old. CAD has a male predilection, with a male-to-female ratio of 5:1 (2).
Clinical presentation
Clinically, the typical patient with CAD is a middle-aged patient who is otherwise in a good state of health, more likely to be male, presenting with new onset of intermittent limb claudication not related to cigarette use or diabetes. The onset of symptoms may be sudden or more insidious. On physical examination, the distal limb pulses may or may not be absent at rest. The Ishikawa’s sign can be seen in CAD, which is the disappearance of the foot pulses with flexion of the knee. This differentiates CAD from the popliteal entrapment syndrome, where the pulse would disappear with contraction of the gastrocnemius during active plantar flexion or passive dorsiflexion of the foot (15). Claudication symptoms in CAD can be transient and may resolve spontaneously (16).
Diagnostic imaging
Ankle-brachial pressure indexes and ankle Doppler flow can be used to demonstrated reduced flow at the ankle, but this finding is nonspecific for CAD (17).
Angiography has traditionally been used for diagnosis of CAD. There are several different appearances of the affected vessels on angiography, such as eccentric narrowing of the lumen (scimitar sign), hourglass narrowing of the lumen, or complete occlusion. Lack of post-stenotic dilatation is a useful sign in CAD (15). However, some cases can have nonspecific complete luminal obstruction during angiography which can be mistaken for an endoluminal lesion (18).
US has become a useful inexpensive, easy and noninvasive diagnostic test for CAD. A thin, echogenic line can be seen on US separating the lumen of the vessel and the cyst, representing the vascular intima and media. The narrowed lumen may present with an ultrasonic scimitar sign on color Doppler imaging. There will be elevated velocity in the narrowed lumen, in cases that are not completely occluded. These findings are considered pathognomonic for CAD on US. Furthermore, US is able to determine the lack of vascular flow in the cyst, and demonstrate posterior acoustic enhancement of the cyst, both of which helps to distinguish CAD from popliteal aneurysms (15).
Computed tomography (CT) and magnetic resonance imaging (MRI) have also been advocated in the diagnosis of CAD (19). On CT, a low attenuating structure can be seen intimately associated with the artery, without intervening fat plane (20). On MRI, the cyst will be seen adjacent the popliteal artery lumen, with characteristic signals of a fluid-filled structure, such as T1 hypointensity, T2 hyperintensity and the lack of flow signal void (17). On either of these two modalities, the cyst will not enhance with the administration of contrast. An additional advantage of using CT for the diagnosis of CAD is the possible subsequently percutaneous aspiration of the cyst under guidance, similar to US, as discussed below.
Management
A number of techniques exist for the treatment of CAD, including surgical intervention, percutaneous aspiration, and percutaneous endovascular intervention. However, the decision to treat should be based on clinical and radiological presentations. Conservative management of CAD should be considered first, as the cysts in CAD may resolve spontaneously, or the patient may not experience significant discomfort to prompt for more invasive treatment options.
Historically, surgical removal was the treatment of choice. Different authors in the past have recommended different techniques, such as complete resection with bypass, excision of the cyst, surgical aspiration of the cyst, or “exarterectomy” where there is circumferential resection of the diseased adventitia (13). With the except of complete resection, most techniques recommend preservation of the native artery.
Percutaneous aspiration is a less invasive, lower risk procedure can be performed using either CT or US guidance, similar to other types of percutaneous fluid collection aspiration. Advantages of the percutaneous procedures include quicker recovery and less risk of adverse effects related to anesthesia (21). Results of this method has been mixed, with some authors reporting success without recurrence, and others reporting recurrence (21,22). Nevertheless, it is our opinion that percutaneous options should be attempted first prior to surgical intervention.
Percutaneous endovascular intervention, such as with angioplasty and stenting have been attempted, with disappointment results. Only one author reported successful treatment in a recurrent case of CAD, with the patient having undergone prior surgical excision. Failure of angioplasty and stenting is attributed to the extraluminal state of the cyst as well as the absence of atherosclerotic process (13,23).
Acknowledgements
We would like to thank Jennifer H. Lee, MFA, for her assistance.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was taken from the patients for reporting this case and any accompanying images.
References
- Atkins HJ, Key JA. A case of myxomatous tumour arising in the adventitia of the left external iliac artery. Br J Surg 1947;34:426. [Crossref] [PubMed]
- Ksepka M, Li A, Norman S. Cystic Adventitial Disease. Ultrasound Q 2015;31:224-6. [Crossref] [PubMed]
- Linquette M, Mesmacque R, Beghin B, et al. Cystic degeneration of the adventitia of the popliteal artery. Apropos of a further case. Sem Hop 1967;43:3005-13. [PubMed]
- Stallworth JM, Brown AG, Burges GE, et al. Cystic adventitial disease of the popliteal artery. Am Surg 1985;51:455-9. [PubMed]
- Levien LJ, Benn CA. Adventitial cystic disease: a unifying hypothesis. J Vasc Surg 1998;28:193-205. [Crossref] [PubMed]
- Tsilimparis N, Hanack U, Yousefi S, et al. Cystic adventitial disease of the popliteal artery: an argument for the developmental theory. J Vasc Surg 2007;45:1249-52. [Crossref] [PubMed]
- Jasinski RW, Masselink BA, Partridge RW, et al. Adventitial cystic disease of the popliteal artery. Radiology 1987;163:153-5. [Crossref] [PubMed]
- Terry JD, Schenken JR, Lohff MR, et al. Cystic adventitial disease. Hum Pathol 1981;12:639-42. [Crossref] [PubMed]
- Haid SP, Conn J, Bergan JJ. Cystic adventitial disease of the popliteal artery. Arch Surg 1970;101:765-70. [Crossref] [PubMed]
- Harris JD, Jepson RP. Cystic degeneration of the popliteal artery. Aust N Z J Surg 1965;34:265-8. [Crossref] [PubMed]
- Desy NM, Spinner RJ. The etiology and management of cystic adventitial disease. J Vasc Surg 2014;60:235-45, 245.e1-11.
- Flanigan DP, Burnham SJ, Goodreau JJ, et al. Summary of cases of adventitial cystic disease of the popliteal artery. Ann Surg 1979;189:165-75. [Crossref] [PubMed]
- Paravastu SC, Regi JM, Turner DR, et al. A contemporary review of cystic adventitial disease. Vasc Endovascular Surg 2012;46:5-14. [Crossref] [PubMed]
- Jones DW, Rezayat C, Winchester P, et al. Adventitial cystic disease of the femoral vein in a 5-year-old boy mimicking deep venous thrombosis. J Vasc Surg 2012;55:522-4. [Crossref] [PubMed]
- Tsolakis IA, Walvatne CS, Caldwell MD. Cystic adventitial disease of the popliteal artery: diagnosis and treatment. Eur J Vasc Endovasc Surg 1998;15:188-94. [Crossref] [PubMed]
- Pursell R, Torrie EP, Gibson M, et al. Spontaneous and permanent resolution of cystic adventitial disease of the popliteal artery. J R Soc Med 2004;97:77-8. [Crossref] [PubMed]
- Elias DA, White LM, Rubenstein JD, et al. Clinical evaluation and MR imaging features of popliteal artery entrapment and cystic adventitial disease. AJR Am J Roentgenol 2003;180:627-32. [Crossref] [PubMed]
- Cassar K, Engeset J. Cystic adventitial disease: a trap for the unwary. Eur J Vasc Endovasc Surg 2005;29:93-6. [Crossref] [PubMed]
- Crolla RM, Steyling JF, Hennipman A, et al. A case of cystic adventital disease of the popliteal artery demonstrated by magnetic resonance imaging. J Vasc Surg 1993;18:1052-5. [Crossref] [PubMed]
- Deutsch AL, Hyde J, Miller SM, et al. Cystic adventitial degeneration of the popliteal artery: CT demonstration and directed percutaneous therapy. AJR Am J Roentgenol 1985;145:117-8. [Crossref] [PubMed]
- Keo HH, Baumgartner I, Schmidli J, et al. Sustained remission 11 years after percutaneous ultrasound-guided aspiration for cystic adventitial degeneration in the popliteal artery. J Endovasc Ther 2007;14:264-5. [Crossref] [PubMed]
- Colombier D, Elias A, Rousseau H, et al. Cystic adventitial disease: importance of computed tomography in the diagnostic and therapeutic management. J Mal Vasc 1997;22:181-6. [PubMed]
- Maged IM, Kron IL, Hagspiel KD. Recurrent cystic adventitial disease of the popliteal artery: successful treatment with percutaneous transluminal angioplasty. Vasc Endovascular Surg 2009;43:399-402. [Crossref] [PubMed]


