Pleurectomy and decortication
Introduction
Macroscopic complete surgical resection with curative intent of malignant mesothelioma includes extrapleural pneumonectomy (EPP) and pleurectomy and decortication (P/D). Various studies have demonstrated decreased short term mortality with P/D compared to EPP as well as improved quality of life and safety in the elderly without increased morbidity and mortality (1-5). This section addresses the pre-operative considerations, operative technique post-operative management and outcome for P/D.
Definitions and operative goals
The International Association for the Study of Lung Cancer (IASLC) performed a multinational survey to help understand current concepts regarding the extent of surgery that is performed for mesothelioma and the nomenclature of those operations (6). P/D was defined as parietal and visceral pleurectomy to remove all gross tumors without diaphragm or pericardial resection. Extended P/D (EPD) was defined as parietal and visceral pleurectomy to remove all gross tumors with resection of the diaphragm and/or pericardium. Although the technique for EPD will be described, ultimately the clinical situation will dictate the extent of resection.
Maximum cytoreduction and adequate lymph node sampling has long been the overall goal of the operation. Previous studies have shown that volume of disease correlates with overall survival (7). In regards to mesothelioma, however, the literature varies regarding what constitutes an adequate or maximum cytoreduction (8-12). Although there exists a completeness of cytoreduction score (CCS) for peritoneal malignancies, there currently does not exist a CCS for mesothelioma.
Pre-operative evaluation
Patient selection for surgery-based treatments can vary between institutions, but the general principles observed by all groups are that the cancer must appear confined to one hemithorax and the patient must be at reasonable risk for the proposed procedure. Histology is also an important determinant in determining operative intervention for mesothelioma, thus a pathologic review of the patient’s specific histology is of critical importance. Many patients with non-epithelioid histology are not considered for surgical intervention due to poor overall prognosis, even though surgical intervention has been observed to prolong survival and improve quality of life (13). The percentage of epithelioid differentiation in biphasic histology was found to be a significant and independent predictor on survival (14). As a result, we do not exclude biphasic histology from P/D, especially if we are convinced we able to improve the patient’s overall quality of life. Purely sarcomatoid histology, however, is not offered operative intervention generally. It is our routine practice that pathologic slides from outside hospitals are re-reviewed by a pathologist at our home institution to both confirm the diagnosis and determine relative amounts of epithelioid vs. sarcomatoid mesothelioma features. We recommend biopsies from different site of the pleura during the diagnostic procedure for adequate evaluation of histology of the tumor (15).
The patient is evaluated to determine if there is disease amenable to surgical resection and to evaluate if the patient has appropriate cardiopulmonary reserve to undergo P/D. Patients selected for P/D are not excluded based on age, as long as they have a good performance status and appropriate cardiopulmonary reserve as determined per pre-operative evaluation. Most patients present with a CT scan of the chest and upper abdomen with intravenous contrast as a means to assess extent of primary tumor and invasion. It is our practice to routinely obtain PET-CT to aid with initial staging of malignant mesothelioma. This helps to evaluate the contralateral hemithorax and abdomen for any possible metastatic disease for which surgical intervention would be contraindicated. We do not routinely perform mediastinoscopy or staging laparoscopy unless suspicious lymphadenopathy in the contralateral hemithorax or suspicious lesions observed in the abdominal cavity on imaging studies.
To assess cardiopulmonary reserve, we routinely obtain complete pulmonary function testing (PFT) with a measurement of the carbon monoxide diffusing capacity of the lung (DLCO). We also obtain a quantitative ventilation and perfusion (V/Q) scan to evaluate differential pulmonary function.
Pre-operative anesthesia discussion
We find it is best to have a select group of anesthesia team (attending and resident) manage the patient during the procedure and a single team for the entire duration of the case if possible. It is anticipated that the patient will be under general anesthesia for 4–6 h and anticipate moderate blood loss often requiring transfusion of packed red blood cells during the surgical procedure. Blood should be immediately available prior to making incision. In regards to vascular access, an arterial line and two large bore (18 gauge) peripheral venous lines are placed. Central venous access is not usually necessary. An epidural catheter is placed pre-operatively for treatment of post-operative pain, but is not utilized during the surgery to avoid any epidural related hypotension.
Ventilator strategy is of upmost importance to the success of the P/D. We require the operative side to be both ventilated and inflated separately from the non-operative lung. Our technique is to use a second, separate ventilator, for the lung on the operative side which uses room air and without any anesthetic vapors in order to avoid fire hazard and to avoid the surgical team inhaling noxious anesthetic vapors. This also helps assist with the actual dissection of the visceral pleura from the lung. Given the large air leaks that are inherently associated with the decortication, the volumes of ventilation of the operative side may have to be kept up to provide adequate inflation, and having a second ventilator provide the inflation without compromising patients oxygenation is beneficial. Ideally, we want the patient to be breathing spontaneously as soon as the decortication is done and soon after the pleural space is closed. Our goal is to have the patient extubated prior to leaving the operating room.
Surgical technique
Preparation, positioning and incision
An epidural is placed pre-preoperatively by the anesthesia team. The patient is then intubated with a dual lumen endotracheal tube. Bronchoscopy is performed to confirm lung isolation. The patient is then positioned in a standard lateral decubitus position.
A lateral thoracotomy is performed. The latissimus muscle is transected, but the serratus anterior muscle is spared. The pleural space is often entered through the sixth intercostal space. Consideration should be given to the location of the bulk of the disease. If the bulk of disease involves the diaphragm, then a lower approach may be necessary and if the bulk is in the apex, then a higher space is desired. Rarely is entry through a second intercostal space is necessary to achieve complete macroscopic resection. Previous biopsy sites or drain site are evaluated and included in the excision as mesothelioma has a propensity to seed previous incisions.
P/D technique
The chest cavity is entered extrapleurally. Deliberate dissection is performed mobilizing the pleura from the chest wall. A self-retaining Finochietto retractor is used to spread the ribs slowly to avoid fracturing the ribs. Blunt hand dissection is performed in the extrapleural plane between the parietal pleural and endothoracic fascia to take down the parietal pleura intact (Figure 1A). We place multiple sponge packs to help tamponade and limit blood loss. It is of paramount importance to move in a systematic, organized fashion throughout the chest in order to limit blood loss. Once the parietal pleura are mobilized, the dissection is continued removing the mediastinal pleura. All mediastinal structures are carefully preserved. On the right side, the superior vena cava, azygous trachea, left and right main bronchi vein and esophagus are carefully protected and on the left side, the esophagus, aorta and vagus nerve, including the recurrent nerves, should be preserved. The phrenic nerves are identified and preserved whenever possible when the diaphragm is not resected. It is also important to appreciate the location of the internal mammary vessels which can inadvertently be injured and be a source of significant blood loss. If they are involved by tumor proper control of the vessels should be achieved and divided.
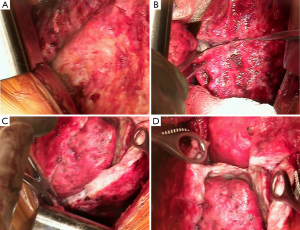
If the tumor involves the diaphragm, either partial or complete resection is performed. Whenever possible, the peritoneal membrane is preserved. Although every effort is made not too enter the peritoneum, this is often very difficult due to the thin nature of the diaphragm at the central tendon and sometimes the tumor involves near full thickness of the diaphragm.
With the parietal pleurectomy now complete, the decortication and resection of the visceral pleura is performed. The anesthesia team is made aware of this transition as this requires a change in ventilation management. A second ventilator is connected to the affected side and the lung is inflated with room air at high tidal volumes (8–10 L/min). A ten blade scalpel is used to cut vertically thru the tumor and past the visceral pleura (Figure 1B). This is perhaps the most crucial step as the visceral pleura must be appropriately identified and removed. The tumor, visceral pleura and parietal pleura are then stripped off the lung using a combination of blunt and sharp dissection. Many techniques are used to achieve this including suction dissection, ultrasound, or plasma, in order to lift the tumor layer off the lung (Figure 1C). Air leaks can be large and are expected during this portion of the procedure. Packing with wet gauze or lap pads is useful to reduce the air leak and encourage formation of a coagulum on the surface of the lung. Due to the tedious nature of this dissection, it is important to continue in a patient, systematic and organized fashion. The dissection is continued in the fissures removing the visceral pleura on both sides towards the pulmonary arterial branches (Figure 1D). Any lymph nodes encountered in the fissure are removed for histological examination.
We next perform the mediastinal lymph node dissection. We routinely remove lymph nodes from levels 4, 7 and 9. It is not unusual to find internal mammary nodes, costophrenic or nodes in the posterior intercostal space which are also removed. We also remove any other nodes that appear abnormal during the dissection. If the patient is a candidate for EPP mediastinal lymph node dissection is conducted earlier with frozen section after the extrapleural mobilization of the parietal pleura, before final decision. If there is any concern for metastatic disease in any of the lymph nodes we then proceed with PD and not EPP in these candidates.
Reconstruction of the diaphragm and pericardium
Reconstruction of the diaphragm, when resected, is always performed. Various materials have been used to reconstruct the diaphragm. We commonly use either 1 or 2 mm Gore-Tex prosthesis. If there are any concerns of infection or patient with long term indwelling catheter with possible colonization, we prefer to use biological material instead of Gore Tex material. The defects are measured and the patch is oversized to repair the diaphragm defect. The prosthetic material is soaked in antibiotic solution prior to implantation. The prosthesis is secured utilizing interrupted and a running non absorbable monofilament suture (#1 prolene or Surgipro) to either the remaining rim of diaphragm or to the ribs. We prefer to leave some macroscopically tumor free diaphragmatic tissue around the inferior vena cava on the right side and the esophagus on the left side if possible, to suture the prosthetic material. The diaphragm is reconstructed at the same level as the native diaphragm or at one or two ribs higher if complete resection of the diaphragm has been performed to assist in dealing with the sub-pulmonary space.
Pericardial replacement varies depending on the extent and location. If substantial portion of the pericardium is resected, or if there is concern for cardiac herniation, the pericardium is replaced with 0.1 mm thick Gore-Tex patch using 3-0 prolene or bioprosthesis if concern for pleural infection or contamination.
Various sealants have been used by other centers and claimed very effective in controlling or reducing lung surface air leak, however, we have not found any effective sealants for this purpose. With the P/D complete, the chest is closed. Two 32 F chest tubes are placed, one anterior and one posterior in the mediastinum. The ribs are approximated with figure-of-8 (#2 Vicryl) and the wound is closed in layers with braided absorbable sutures.
The epidural analgesia is started before extubation of the patient. Patient is recovered from anesthesia and generally extubated in the operating room. The chest tubes are placed to water seal while intubated and with the patient on positive pressure ventilation. Once extubated, the chest tubes are placed to −10 mmHg of underwater seal wall suction.
Post-operative care
In PACU or in the ICU, laboratory values of complete blood count and basic metabolic panel are obtained as well as a baseline chest X-ray. All of our patients are managed in the ICU for post-operative monitoring of vitals, respiratory status and urine output after the operation. The pleural space is monitored by serial chest X-rays as indicated and the chest tubes are placed to water seal once the pleural space is stable, typically around post-operative day 2 or 3, and subsequently removed once the air leak has resolved. If the patient persists with air leak and the pleural space is stable, it is not unusual for us to discharge patient home with portable system.
Outcomes
A recent meta-analysis by Taioli and colleagues evaluated survival after pleurectomy decortication versus EPP (3). A literature review evaluating reports in the last 10 years of outcomes after pleurectomy decortication demonstrates a median survival between 10 and 25 months (Table 1) (2,5,9,12,16-25). Thirty-day mortality was between 0.0–6.8% across all studies. The most frequent major complications were respiratory failure (2.3–7.1%) and bleeding (0.0–16.7%). We believe that careful attention to the intraoperative ventilator strategy and systematic approach to bleeding during the procedure can help decrease this complication rate. Minor complications included arrhythmia, between 2.3–21.4%, and were described most frequently as a supraventricular tachycardia or atrial fibrillation. Prolonged post-operative air leaks were between 7.1–23.5% of patients in reports when described.
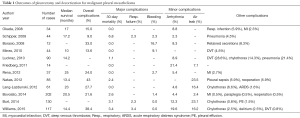
Full table
In regards to quality of life, P/D has been found to be associated with improved quality of life compared to EPP as patients had significant improvement in both fatigue and dyspnea symptoms after undergoing P/D this has been validated in a larger cohort (4). P/D has also been found to be a safe procedure to perform in the elderly (70 years old and older) without increased morbidity or mortality or short term survival (5).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: Results in 663 patients. J Thorac Cardiovasc Surg 2008;135:620-6. [Crossref] [PubMed]
- Burt BM, Cameron RB, Mollberg NM, et al. Malignant pleural mesothelioma and the Society of Thoracic Surgeons Database: An analysis of surgical morbidity and mortality. J Thorac Cardiovasc Surg 2014;148:30-5. [Crossref] [PubMed]
- Taioli E, Wolf AS, Flores RM. Meta-Analysis of Survival After Pleurectomy Decortication Versus Extrapleural Pneumonectomy in Mesothelioma. Ann Thorac Surg 2015;99:472-80. [Crossref] [PubMed]
- Mollberg NM, Vigneswaran Y, Kindler HL, et al. Quality of Life After Radical Pleurectomy Decortication for Malignant Pleural Mesothelioma. Ann Thorac Surg 2012;94:1086-92. [Crossref] [PubMed]
- Williams T, Duraid H, Watson S, et al. Extended Pleurectomy and Decortication for Malignant Pleural Mesothelioma Is an Effective and Safe Cytoreductive Surgery in the Elderly. Ann Thorac Surg 2015;100:1868-74. [Crossref] [PubMed]
- Rice D. Standardizing surgical treatment in malignant pleural mesothelioma. Ann Cardiothorac Surg 2012;1:497-501. [PubMed]
- Kircheva DY, Husain AN, Watson S, et al. Specimen weight and volume: important predictors of survival in malignant pleural mesothelioma. Eur J Cardiothorac Surg 2016;49:1642-7. [Crossref] [PubMed]
- Richards WG, Zellos L, Bueno R, et al. Phase I to II study of pleurectomy/decortication and intraoperative intracavitary hyperthermic cisplatin lavage for mesothelioma. J Clin Oncol 2006;24:1561-7. [Crossref] [PubMed]
- Friedberg JS, Mick R, Culligan M, et al. Photodynamic Therapy and the Evolution of a Lung-Sparing Surgical Treatment for Mesothelioma. Ann Thorac Surg 2011;91:1738-45. [Crossref] [PubMed]
- Rusch V, Saltz L, Venkatraman E, et al. A phase II trial of pleurectomy/decortication followed by intrapleural and systemic chemotherapy for malignant pleural mesothelioma. J Clin Oncol 1994;12:1156-63. [PubMed]
- Pass HI, Temeck BK, Kranda K, et al. Phase III randomized trial of surgery with or without intraoperative photodynamic therapy and postoperative immunochemotherapy for malignant pleural mesothelioma. Ann Surg Oncol 1997;4:628-33. [Crossref] [PubMed]
- Lang-Lazdunski L, Bille A, Lal R, et al. Pleurectomy/decortication is superior to extrapleural pneumonectomy in the multimodality management of patients with malignant pleural mesothelioma. J Thorac Oncol 2012;7:737-43. [Crossref] [PubMed]
- Yan TD, Boyer M, Tin MM, et al. Prognostic Features of Long-term Survivors After Surgical Management of Malignant Pleural Mesothelioma. Ann Thorac Surg 2009;87:1552-6. [Crossref] [PubMed]
- Vigneswaran WT, Kircheva DY, Ananthanarayanan V, et al. Amount of Epithelioid Differentiation is a Predictor of Survival in Malignant Pleural Mesothelioma. Ann Thorac Surg 2017;103:962-6. [Crossref] [PubMed]
- Bueno R, Reblando J, Glickman J, et al. Pleural Biopsy: A Reliable Method for Determining the Diagnosis But Not Subtype in Mesothelioma. Ann Thorac Surg 2004;78:1774-6. [Crossref] [PubMed]
- Rosenzweig KE, Fox JL, Zelefsky MJ, et al. A pilot trial of high-dose-rate intraoperative radiation therapy for malignant pleural mesothelioma. Brachytherapy 2005;4:30-3. [Crossref] [PubMed]
- Okada M, Mimura T, Ohbayashi C, et al. Radical surgery for malignant pleural mesothelioma: results and prognosis. Interact Cardiovasc Thorac Surg 2008;7:102-6. [Crossref] [PubMed]
- Schipper PH, Nichols FC, Thomse KM, et al. Malignant Pleural Mesothelioma: Surgical Management in 285 Patients. Ann Thorac Surg 2008;85:257-64. [Crossref] [PubMed]
- Borasio P, Berruti A, Billé A, et al. Malignant pleural mesothelioma: clinicopathologic and survival characteristics in a consecutive series of 394 patients. Eur J Cardiothorac Surg 2008;33:307-13. [Crossref] [PubMed]
- Mineo TC, Ambrogi V, Cufari ME, et al. May cyclooxygenase-2 (COX-2), p21 and p27 expression affect prognosis and therapeutic strategy of patients with malignant pleural mesothelioma? Eur J Cardiothorac Surg 2010;38:245-52. [Crossref] [PubMed]
- Luckraz H, Rahman M, Patel N, et al. Three decades of experience in the surgical multi-modality management of pleural mesothelioma. Eur J Cardiothorac Surg 2010;37:552-6. [Crossref] [PubMed]
- Rena O, Casadio C. Extrapleural pneumonectomy for early stage malignant pleural mesothelioma: A harmful procedure. Lung Cancer 2012;77:151-5. [Crossref] [PubMed]
- Nakas A, von Meyenfeldt E, Lau K, et al. Long-term survival after lung-sparing total pleurectomy for locally advanced (International Mesothelioma Interest Group Stage T3-T4) non-sarcomatoid malignant pleural mesothelioma. Eur J Cardiothorac Surg 2012;41:1031-6. [Crossref] [PubMed]
- Nakas A, Waller D, Lau K, et al. The new case for cervical mediastinoscopy in selection for radical surgery for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2012;42:72-6. [Crossref] [PubMed]
- Bovolato P, Casadio C, Billè A, et al. Does surgery improve survival of patients with malignant pleural mesothelioma?: a multicenter retrospective analysis of 1365 consecutive patients. J Thorac Oncol 2014;9:390-6. [Crossref] [PubMed]


