Coeliac disease and the videocapsule: what have we learned till now
Introduction
Celiac disease affects approximately 1% of the worldwide population (1). Many patients with celiac disease go undiagnosed, even in developed countries, owing to the difficulty in making a diagnosis (2). Generally, serum antibody tests are used in screening for celiac disease (3). However, these tests may occasionally be falsely positive or negative (4). The reference standard for diagnosis is biopsy to determine the presence of small bowel (SB) villous atrophy (5). The proximal duodenum is most commonly affected by the disease; however, patients generally also have atrophy in the jejunal or ileal mucosa. Villous atrophy is graded by analyzing histopathologic changes using light microscopy (6). Therefore, videocapsule endoscopy (VCE) can be used to image more distal regions of the SB where atrophy may occur, although biopsy cannot yet be obtained with any of the commercially available VCE devices (7). Nevertheless, the resolution of both standard endoscopy and VCE is improving, which is useful to more carefully detail the small intestinal mucosal surface for determining the presence of pathologic alterations. The gross macroscopic features include the presence of protrusions, fissuring of the surface, a mottled appearance, and scalloping of the mucosal folds (see Figures 1,2) (8,9).
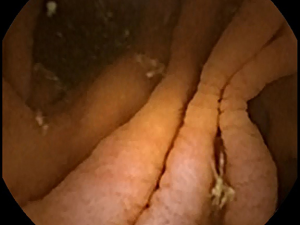
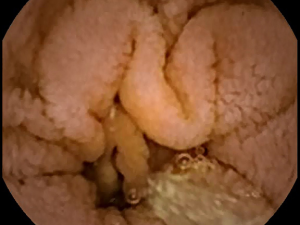
An important quantitative problem in celiac disease research is to determine areas where pathology may occur, that can then be biopsied or flagged for closer inspection. Villous atrophy is often patchy and subtle in gross macroscopic appearance. Therefore it can be readily missed (10). There may be a tentative diagnosis based on antibody serology, as well as genetic tests, but at present these are not considered definitive. Furthermore, in some patients there may be no evident villous atrophy (11). For proof that some untreated celiac patients lack villous atrophy, careful high-resolution analysis of the entire SB mucosal surface is required.
Celiac patients react to gluten and its components, a protein found in wheat, rye, and barley grains (12). The current treatment remains maintenance on a gluten-free diet (GFD). The villi of the SB often heal, though this may require months or even years, and may never be complete (13). Systemic manifestations of the disease, in which there is autoimmune reactivity to transglutaminase in other organs, can also reduce or completely resolve when patients maintain a GFD (14). To check progress of mucosal healing during treatment, serum antibody tests and follow-up endoscopy with biopsy are utilized. When gross structural changes occur including the disappearance of mucosal crevices, reduction in the mottled appearance and/or in the scalloping of mucosal folds, and lesser degree of villous atrophy, as much as they can be visualized, as well as improvement in indices of villous atrophy under light microscopy, these indicate progress in reducing or eliminating the autoimmune reaction.
In this work, some of the quantitative methods for analyzing VCE images for presence of pathology such as villous atrophy are reviewed, and possible future foci and directions for analysis are discussed. By improving the quantitative analysis of endoscopic images, along with the increasing temporal and spatial resolution of these images (15), it should be possible to detect subtle, patchy presence of pathology in the SB mucosa, and to improve the grading system so that progress on a GFD can be more accurately gauged.
Methods
Clinical protocol
For the images shown in this review, VCE data were obtained retrospectively from celiac patients prior to or within 3 months of onset of a GFD. In these patients, a diagnostic biopsy was obtained prior to onset of the GFD. Light microscopic analysis showed Marsh grade II-IIIC lesions. VCE data was also obtained retrospectively from control (non-celiac) patients lacking villous atrophy. All of the patients were evaluated at Columbia University Medical Center, New York. Informed consent was obtained from all patients prior to VCE. The indications for the VCE procedure were screening for celiac disease and Crohn’s disease, bleeding of unknown origin, iron deficient anemia, and chronic diarrhea. Pediatric patients, pregnant women, and patients with a history of intestinal obstruction, presence of a pacemaker, or chronic use of non-steroidal anti-inflammatory drugs (NSAIDs) were excluded from the study. For analysis, only complete VCE studies, reaching the colon, were used. Retrospective analysis of VCE data was approved by the Internal Review Board of Columbia University Medical Center.
Data acquisition
The PillCam® SB2 capsule that was used for imaging was manufactured by Given Imaging® , Yokneam, Israel (now part of Medtronic, Inc.) (16). The system includes a recorder with harness and battery pack with charger, a real-time viewer, antenna lead set, and connecting cable. The capsule measures 26 mm × 11 mm in dimension, and the frame rate is two digital images per second. All subjects swallowed the PillCam® SB2 capsule, having a radio transmitter, following a 12-h fast, and wore a small portable recording device. The recorder received radioed images via a sensor array that transmitted signals acquired by the capsule camera as it passed through the GI tract. The procedure began early in morning by swallowing the capsule with approximately 200 mL of water, and investigation was terminated either upon arrival of the capsule in the cecum, or after eight hours. Subjects were allowed to drink water two hours after ingesting the capsule, and to eat a small meal after four hours. Videos were reviewed and interpreted by an experienced gastroenterologist using an HIPAA-compliant PC-based workstation equipped with analysis software. Videos were then exported for further analysis. Videoclips of 200 frames each were acquired from five locations in the SB of each patient by the treating physicians. The five regions were: (I) the duodenal bulb; (II) distal duodenum; (III) jejunum; (IV) proximal ileum and (V) distal ileum. The recorded digital information was then offloaded to computer console, and videos from the small intestine were analyzed retrospectively.
For simplicity, measurements are often done using grayscale level images. To obtain grayscale images from color videoclips, a converting process is needed. Matlab code is often used for this purpose (17,18). MPG files, obtained upon acquisition of the videocapsule data without identifiers, were converted to PGM images, which have a straightforward format that can be easily utilized for analysis. The MPG files could be played in movie form to show capsule progress over a period of 50–100 sec. The spatial resolution of all acquired images was 576×576 pixels. In terms of the actually distances that the image covers on the mucosal surface being imaged, this will depend upon camera distance and angle to the surface.
Measurements
There are several methods to detect pathology in the small intestinal mucosa of celiac disease patients. In this section, measurements made on VCE image series (videoclips) will be explored.
Textural components
Texture is often obtained using grayscale images, with gray values ranging from 0 (black) to 255 (white) (19,20). A commonly used measure of texture is the standard deviation or its square, the variance statistic (18). An important question to answer is to address how spatial and temporal differences in texture should be differentiated. Each endoscopic image likely encompasses an area of a few square centimeters, although this depends on the camera distance and angle from the mucosal surface. Moreover, if the camera angle is not normal to the mucosal surface, which is quite probably the case, then the spatial resolution will vary throughout the image, i.e., distortion of the field occurs. As a first approximation, each image can be treated as a single entity, with texture computed in the same manner over the entire image. Thus a single value of texture is obtained for each image. Alternatively, subimages can be formed (19-22), for example of fixed 10×10 pixel areas. Over the 576×576 pixel image, there are 57×57 such subimages, with edges of 6 pixels not used. The use of a single quantifier for the entire image can be problematic, because detailed features that might be present are averaged. Yet, the use of fixed-location subimages can also be problematic, since any particular subimage may not necessarily capture the unique content indicating pathology, and additionally there may be overlap of pathologic and non-pathologic areas in a single subimage. However, use of these metrics can be helpful to obtain a global picture of the textural characteristics present in any particular image. If there is substantial pathology, it will be reflected in both of the aforementioned metrics (global and subimage regions). It would be expected that pathologic regions would be represented as areas of greater texture as compared with the relative uniformity of normal zones of the small intestinal mucosa. This would then be a working hypothesis used to analyze these regions, with the above-described textural descriptors or other textural descriptors.
An additional concern to be addressed in analyzing the VCE image series is that all images in the set require consideration. Suppose that the variance descriptor is used to measure the texture of each image, and suppose it is a single, global (i.e., image-wide) measurement, rather than being measured separately in subimages. If there are 200 images in the videoclip series, then there will be 200 such variance measurements, one for each image in the series. One way to characterize the series then, is by calculating the mean and standard deviation of the variance textural descriptor over the N=200 images in the series (23,24). If villous atrophy is present and it is patchy, thus being evident in some but not all images in the series, then a larger value would be expected for the mean (i.e., greater texture since pathology is present) and a larger value also for the standard deviation (i.e., greater variation in texture) as compared to videoclip series from control patients lacking pathology.
Spectral analysis
Spectral analysis can be useful to detect periodicities in videocapsule image series (22). Any such periodicity may arise from peristalsis as well as from fluctuations in the capsule orientation, and also from alterations in the degree of pathology as the capsule moves distally through the small intestine. When spectra of the image series are obtained, they represent points in both time and space. Each image is obtained at a 0.5 second increment in time. The average distance the capsule travels distally along the small intestine in that interval is variable, but on the order of millimeters. Moreover, it is possible for the capsule to stall or even to move retrograde for some time, depending on the motion of the small intestinal wall in proximity to the capsule. More recently, capsules have been designed to update the image snapshot with variable time increment (25). Sensors within the capsule estimate motion, and fewer images per time are acquired when the capsule is moving more slowly or is stalled in its location. Conversely, this videocapsule obtains images more rapidly when the distal velocity increases. For simplicity, the average image grayscale level measured for all images in the series, can be used to generate a frequency spectrum. The spectrum would be expected to show any periodicities in the average gray level over 200 frames (100 seconds time interval). Spectral peaks indicating periodicity would be anticipated to occur for example, due to peristalsis. Each time a contractile wave passes the capsule, it may cause it to orient itself in one direction, i.e., along the local long axis of the small intestine, due to the constriction process. The view along the long axis of the small intestine usually includes darker areas which are farther from the capsule. After the peristaltic wave passes, the capsule would be freer to orient randomly, and would be more likely to turn toward the mucosal wall.
The variance textural descriptor can similarly be used to generate frequency spectra (22). Obtained by measurement over the entire image, there is again one such descriptor per image, and 200 measurements are made over the 200 image series. This descriptor would be expected to detect any periodicity in the degree of patchiness of the villous atrophy or other pathology. Were a particular image to be composed of patchy villous atrophy, the variance descriptor would be expected to be large in magnitude. For other images, lacking villous atrophy, the variance descriptor would be expected to be lesser in magnitude since the normal villi and the overall mucosal structure would be more uniform. Variation from one image to the next, would cause spectral peaks to be generated. Conversely, if there is uniform pathology and texture throughout the image, the variance descriptor would be smaller in magnitude. Other areas, in other images, having normal villi, would also have low values for the variance descriptor due to uniformity. Thus in this case, few spectral peaks would be expected to be evident. However, by using the mean textural descriptor, large values for mean grayscale level would be anticipated to be measured where pathology is evident and uniform throughout a particular image, and smaller mean grayscale level values would be expected in images with normal villi; thus in this case peaks in the frequency spectrum would likely be more prominent. The presence of spectral peaks represents periodicity (when a single or a few spectral peaks are present), but this is also evidence of high variation in measured values from one image to the next, without substantial periodicity in the variability (generating many spectral peaks).
Motility
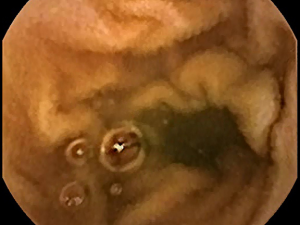
Currently, methods are being developed to measure actual motility in the small intestine directly. However, indirect measures of motility can presently be obtained from videocapsule image series (26). As the intestinal wall becomes more distant from the capsule lens, the acquired image becomes darker in proportion to the inverse square law of light. Within an image, the camera view along the long axis of the small intestine is always darker for this reason. The mucosal surface viewed along the long axis is relatively distant from the camera lens. These areas tend to be the darkest areas in the image (Figure 3). Movement of the capsule away from the long axis, and movement of the intestinal walls along the long axis, will both alter the amount of distant, dark surface in the camera image view. This phenomenon can be used as an indirect measure of small intestinal motility (26). One metric that can be extracted for this purpose is the dark surface area in the image, with a threshold grayscale level used to detect very dark (and usually distant) surfaces (grayscale values lower than threshold are selected). Another metric that can be extracted is the centroid (x- and y-axis centers) of the dark region. One further metric would be the maximal width of the dark region. Over the image series, the variation of the area, maximal width, and centroid location are indirect indicators of the degree of motility of the small intestine in proximity to the capsule. Variations in these parameters are indicative of differences in motility. This information is potentially useful to discern areas with versus those without villous atrophy or other pathology in the small intestine (26).
Results
Visual methods
With the increasing spatial resolution now becoming available for videocapsule data acquisition, it will be possible to detect villous atrophy by visual inspection of VCE images (27,28). More macroscopic alterations in the mucosa including scalloping, mosaicism and reduced folds can also be observed in and can be assistive for diagnosis of celiac disease (29). Other groups have similarly found VCE to be helpful in determining the presence of villous atrophy in suspected celiac disease patients (30-32). VCE can also be implemented to evaluate celiac disease in the settings of ongoing villous atrophy even after GFD treatment (33).
Videocapsule quantitative features
Computer-aided decision (CAD) is emerging to assist in the detection of villous atrophy during screening for celiac disease with VCE (34). With present-day technology, it is now possible to improve the endoscopic detection of intestinal mucosa alterations due to celiac disease using an expert system combined with textural analysis (35). Local binary pattern (LBP) operators and wavelet transform subbands are both useful to subdivide regions of atrophy into a four-class system according to the Marsh score (36). Narrow-band imaging (NBI), which utilizes blue and green wavelengths of light for illumination, can be incorporated for contrast enhancement of vascular patterns on the mucosal surface (37). It is also possible to inspect multiple mucosal layers simultaneously for the presence of pathology, using confocal laser endomicroscopy, also known as visual biopsy (38). The scale invariance property of multi-scale and multi-orientation wavelet transforms can also be applied for texture recognition and classification of pathologic regions in VCE images acquired from untreated celiac disease patients (39). Distortion-correction to account for viewing angle, when applied to endoscopic duodenal imagery, has been tried; however it does not readily improve automated classification of the celiac disease affected mucosal patches.
Videocapsule quantitative characteristics
Untreated celiac patients with villous atrophy in the small intestine tend to have significantly greater texture, in terms of the variance descriptor as compared with controls lacking villous atrophy (19,20). Furthermore, celiac imaging data tends to exhibit significantly greater variation in the mean value textural descriptor over the image series as compared with controls. In celiac patients, the dominant spectral peak (i.e., the tallest spectral peak in physiologic range) is of significantly lower frequency in untreated patients with celiac disease versus controls (26). These descriptors can be utilized to construct nonlinear discriminant functions for classification of untreated patients with celiac disease versus controls, with improved sensitivity and specificity.
Another form of analysis utilizes basis images, which are reconstructed VCE images with salient features reinforced (40,41). Images with villous atrophy, obtained from patients with untreated celiac, tend to show greater variation in the mean gray level texture descriptor over the videoclip image series as compared with control patients with no villous atrophy. Moreover, an increased mean gray level texture variation observed in untreated celiac patients has been correlated with a lower frequency of the dominant spectral peak. There is also a significant correlation between an increased variance texture descriptor observed in patients with untreated celiac and a lower frequency of the dominant spectral peak. Thus celiac patients tend to have substantial variation in texture over image series, and lower frequency component values (indicative of longer periodicity) as compared with control individuals without SB pathology.
In terms of indirect motility measurements obtained by thresholding the lowest grayscale VCE image pixels, this metric has been found useful to distinguish untreated celiac patient VCE imaging data versus controls (26). Using the standard deviation of the xy centroid and the mean and standard deviation of the maximal width, celiac patient data can be distinguished from controls using a three-dimensional (3D) scatterplot, with sensitivities and specificities greater than 90% at most of the defined regions along the SB. A nonlinear discriminant function is again utilized in each case to solve the two-class problem. Thus, several descriptors are currently used for detecting and characterizing villous atrophy from VCE imaging.
Discussion
Summary
In this review, methods used to the present day, for quantitative detection and analysis of villous atrophy in the small intestinal mucosa of celiac disease patients using VCE images series were described and discussed.
Deficiencies in current methods of videocapsule image measurement
Although the results that were reviewed herein are promising, there are potential drawbacks to all of the described methods. A main drawback is in the data acquisition component of the analysis—areas with subtle if any pathology, not readily detectable by visual inspection or computerized means, but in areas of biopsy-proven villous atrophy, should be used as a suitable challenge of the efficacy of the methodology in future studies. Thus the images used for analysis of untreated celiac patients would then more likely have only subtle differences in textural magnitude and variation, and subtle variation in other quantitative features, both within each image and over an image series. This is important to improve the technology over the present day. Control patient data on the other hand, should consist of images with suspected pathologic regions, caused by other than villous atrophy, which do not necessarily have a uniform and smooth appearance. These latter images would be anticipated to possess properties of textural magnitude and variation more similar to those observed in untreated celiac patient images, making the differentiation between these two classes more difficult. This is also important to improve the technology over what is currently available.
A further challenge for future work in videocapsule analysis would be to analyze untreated celiac images without any evidence of pathology in the gross macroscopic structure, throughout the small intestine. Some celiac patients have a villous atrophy grade of 2 or less, meaning that at the microscopic level, differences in cellular properties are apparent, but there may be little or no macroscopically evident changes in the structure of the intestinal villi. By developing means to detect minute or subtle structural changes via videocapsule endoscopy in these regions, celiac disease could become detectable in cases where it would otherwise be missed. Furthermore, it is unclear at present whether untreated celiac patients who seem to lack any small intestinal villous atrophy on biopsy might in fact have villous atrophy that has been missed due to subtlety and patchiness. The question of whether there are untreated celiac patients without any villous atrophy remains unanswered.
Moreover, a major drawback to videocapsule endoscopy is the frequent presence of extraneous and opaque substances and air bubbles in the field of view (Figure 4). It is possible to use edge detection methodology to find areas with air bubbles and mask them (42). This can be of major concern since if left within the image, the appearance of air bubbles potentially invalidates many types of feature measurements and particularly, textural descriptors. The presence of extraneous and opaque substances can likewise alter measurements and obscure features important to the detection and characterization of villous atrophy. Any method utilized for recognition of these unwanted materials should include a paradigm to mask them, and to account for the resulting decrease in field-of-view in each image when tabulating measurement data and comparing image-to-image changes.

Conclusions
For quantitative analysis of videocapsule endoscopy images, textural descriptors are the mainstay in the quantitative detection and characterization of villous atrophy. However, frequency analysis and motility estimates have also been successfully used to determine differences in pathologic versus normal regions, as measured from videocapsule endoscopic image series. Furthermore, enhancements in the available acquisition and visualization methods for this purpose, have led to the possibility of detecting villous atrophy by visual inspection when an expert observer peruses the image series. Use of visual inspection is potentially desirable for straightforward detection of pathology, but this modality is potentially limited by observer bias and the possibility of missed regions of atrophy. Other enhancements likely to be of great importance in future studies include the use of scale-invariant features and measures to reduce distortion caused by viewing angle and artifacts, and distortion correction methods (43).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Green PH, Lebwohl B, Greywoode R. Celiac disease. J Allergy Clin Immunol 2015;135:1099-106. [Crossref] [PubMed]
- Green PH. Where are all those patients with Celiac disease? Am J Gastroenterol. 2007;102:1461-3. [Crossref] [PubMed]
- Green PH, Jabri B. Coeliac disease. Lancet 2003;362:383-91. [Crossref] [PubMed]
- Murray JA, Herlein J, Mitros F, et al. Serologic testing for celiac disease in the United States: results of a multilaboratory comparison study. Clin Diagn Lab Immunol 2000;7:584-7. [PubMed]
- Green PH, Rostami K, Marsh MN. Diagnosis of coeliac disease. Best Pract Res Clin Gastroenterol 2005;19:389-400. [Crossref] [PubMed]
- Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol 1999;11:1185-94. [Crossref] [PubMed]
- Rokkas T, Niv Y. The role of video capsule endoscopy in the diagnosis of celiac disease: a meta-analysis. Eur J Gastroenterol Hepatol 2012;24:303-8. [Crossref] [PubMed]
- Ciaccio EJ, Bhagat G, Lewis SK, et al. Quantitative image analysis of celiac disease. World J Gastroenterol 2015;21:2577-81. [Crossref] [PubMed]
- Ciaccio EJ, Bhagat G, Lewis SK, et al. Suggestions for automatic quantitation of endoscopic image analysis to improve detection of small intestinal pathology in celiac disease patients. Comput Biol Med 2015;65:364-8. [Crossref] [PubMed]
- Dewar DH, Ciclitira PJ. Clinical features and diagnosis of celiac disease. Gastroenterology 2005;128:S19-24. [Crossref] [PubMed]
- Kaukinen K, Mãki M, Partanen J, et al. Celiac disease without villous atrophy. Dig Dis Sci 2001;46:879-87. [Crossref] [PubMed]
- Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity ('celiac sprue'). Gastroenterology 1992;102:330-54. [Crossref] [PubMed]
- Lebwohl B, Granath F, Ekbom A, et al. Mucosal healing and mortality in coeliac disease. Aliment Pharmacol Ther 2013;37:332-9. [Crossref] [PubMed]
- Dieterich W, Ehnis T, Bauer M, et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med 1997;3:797-801. [Crossref] [PubMed]
- Pohl J, Aschmoneit I, Schuhmann S, et al. Computed image modification for enhancement of small-bowel surface structures at video capsule endoscopy. Endoscopy 2010;42:490-2. [Crossref] [PubMed]
- Metzger YC, Adler SN, Shitrit AB, et al. Comparison of a new PillCam™ SB2 video capsule versus the standard PillCam™ SB for detection of small bowel disease. Reports in Medical Imaging 2009;2:7-11. [Crossref]
- Ciaccio EJ, Tennyson CA, Bhagat G, et al. Use of shape-from-shading to estimate three-dimensional architecture in the small intestinal lumen of celiac and control patients. Comput Methods Programs Biomed 2013;111:676-84. [Crossref] [PubMed]
- Ciaccio EJ, Tennyson CA, Bhagat G, et al. Implementation of a polling protocol for predicting celiac disease in videocapsule analysis. World J Gastrointest Endosc 2013;5:313-22. [Crossref] [PubMed]
- Ciaccio EJ, Tennyson CA, Lewis SK, et al. Distinguishing patients with celiac disease by quantitative analysis of videocapsule endoscopy images. Comput Methods Programs Biomed 2010;100:39-48. [Crossref] [PubMed]
- Ciaccio EJ, Tennyson CA, Bhagat G, et al. Classification of videocapsule endoscopy image patterns: comparative analysis between patients with celiac disease and normal individuals. Biomed Eng Online 2010;9:44. [Crossref] [PubMed]
- Ciaccio EJ, Bhagat G, Tennyson CA, et al. Quantitative assessment of endoscopic images for degree of villous atrophy in celiac disease. Dig Dis Sci 2011;56:805-11. [Crossref] [PubMed]
- Ciaccio EJ, Tennyson CA, Bhagat G, et al. Robust spectral analysis of videocapsule images acquired from celiac disease patients. Biomed Eng Online 2011;10:78. [Crossref] [PubMed]
- Ciaccio EJ, Bhagat G, Lewis SK, et al. Extraction and processing of videocapsule data to detect and measure the presence of villous atrophy in celiac disease patients. Comput Biol Med 2016;78:97-106. [Crossref] [PubMed]
- Ciaccio EJ, Bhagat G, Lewis SK, et al. Recommendations to quantify villous atrophy in videocapsule endoscopy images of celiac disease patients. World J Gastrointest Endosc 2016;8:653-62. [Crossref] [PubMed]
- Fisher LR, Hasler WL. New vision in video capsule endoscopy: current status and future directions. Nat Rev Gastroenterol Hepatol 2012;9:392-405. [Crossref] [PubMed]
- Ciaccio EJ, Tennyson CA, Bhagat G, et al. Quantitative estimates of motility from videocapsule endoscopy are useful to discern celiac patients from controls. Dig Dis Sci 2012;57:2936-43. [Crossref] [PubMed]
- Murray JA, Rubio-Tapia A, Van Dyke CT, et al. Mucosal atrophy in celiac disease: extent of involvement, correlation with clinical presentation, and response to treatment. Clin Gastroenterol Hepatol 2008;6:186-93. [Crossref] [PubMed]
- Spada C, Riccioni ME, Urgesi R, et al. Capsule endoscopy in celiac disease. World J Gastroenterol 2008;14:4146-51. [Crossref] [PubMed]
- Chang MS, Rubin M, Lewis SK, et al. Diagnosing celiac disease by video capsule endoscopy (VCE) when esophagogastroduodenoscopy (EGD) and biopsy is unable to provide a diagnosis: a case series. BMC Gastroenterol 2012;12:90. [Crossref] [PubMed]
- Petroniene R, Dubcenco E, Baker JP, et al. Given capsule endoscopy in celiac disease: evaluation of diagnostic accuracy and interobserver agreement. Am J Gastroenterol 2005;100:685-94. [Crossref] [PubMed]
- Hopper AD, Sidhu R, Hurlstone DP, et al. Capsule endoscopy: an alternative to duodenal biopsy for the recognition of villous atrophy in coeliac disease? Dig Liver Dis 2007;39:140-5. [Crossref] [PubMed]
- Rondonotti E, Spada C, Cave D, et al. Video capsule enteroscopy in the diagnosis of celiac disease: a multicenter study. Am J Gastroenterol 2007;102:1624-31. [Crossref] [PubMed]
- Spencer M, Baker J, Azeem A, et al. Video capsule endoscopy in the evaluation of celiac patients with persistent or recurrent symptoms. Who and when? International Journal of Celiac Disease 2016;4:55-60.
- Hegenbart S, Uhl A, Vécsei A. Survey on computer aided decision support for diagnosis of celiac disease. Comput Biol Med 2015;65:348-58. [Crossref] [PubMed]
- Gadermayr M, Kogler H, Karla M, et al. Computer-aided texture analysis combined with experts' knowledge: Improving endoscopic celiac disease diagnosis. World J Gastroenterol 2016;22:7124-34. [Crossref] [PubMed]
- Vécsei A, Amann G, Hegenbart S, et al. Automated marsh-like classification of celiac disease in children using local texture operators. Comput Biol Med 2011;41:313-25. [Crossref] [PubMed]
- Emura F, Saito Y, Ikematsu H. Narrow-band imaging optical chromocolonoscopy: advantages and limitations. World J Gastroenterol 2008;14:4867-72. [Crossref] [PubMed]
- Leong RW, Nguyen NQ, Meredith CG, et al. In vivo confocal endomicroscopy in the diagnosis and evaluation of celiac disease. Gastroenterology 2008;135:1870-6. [Crossref] [PubMed]
- Hegenbart S, Uhl A, Vécsei A, et al. Scale invariant texture descriptors for classifying celiac disease. Med Image Anal 2013;17:458-74. [Crossref] [PubMed]
- Ciaccio EJ, Tennyson CA, Bhagat G, et al. Methods to quantitate videocapsule endoscopy images in celiac disease. Biomed Mater Eng 2014;24:1895-911. [PubMed]
- Ciaccio EJ, Tennyson CA, Bhagat G, et al. Transformation of videocapsule images to detect small bowel mucosal differences in celiac versus control patients. Comput Methods Programs Biomed 2012;108:28-37. [Crossref] [PubMed]
- Suenaga M, Fujita Y, Hashimoto S, et al. A method of bubble removal for computer-assisted diagnosis of capsule endoscopic images. In: International Conference on Industrial, Engineering and Other Applications of Applied Intelligent Systems. Springer International Publishing, 2014:228-33.
- Hämmerle-Uhl J, Höller Y, Uhl A, et al. Endoscope distortion correction does not (easily) improve mucosa-based classification of celiac disease. Med Image Comput Comput Assist Interv 2012;15:574-81. [PubMed]


