Extrapleural pneumonectomy (EPP) vs. pleurectomy decortication (P/D)
Introduction
The technique of tumor resection for malignant pleural mesothelioma (MPM) is one of the most debated topics in thoracic surgery. Pleuropneumonectomy was first applied in MPM by Dr. Butchart and reported the results of 29 patients in 1976 (1). En bloc diaphragm and pericardial resection was performed in most cases. In five early cases, diaphragm was not completely excised. Diaphragm and pericardial reconstruction was performed in 24 and 15 patients respectively. Hospital mortality was 31% and only 3 patients survived 2 years or longer, reaching 3, 5 and 6 years. The significance of histologic subtype in the course of the disease was also evident at that time with the following statement:
“Small numbers make statistical analysis unhelpful but it is interesting to note that 100% of mesenchymal tumors had already reached stage IV at the time of death, whereas two of epithelial tumors (28.6%) were still in stage I in spite of long histories”.
The peri- and post-operative results of pleuropneumonectomy/extrapleural pneumonectomy (EPP) were improved in later decades and this also translated to a better survival rate in long term.
Pleurectomy/decortication (P/D) is one of the first surgical techniques defined in Thoracic surgery and has been used extensively for palliation of MPM. One of the first reports was from Memorial Sloan Kettering Center and in 17 patients who underwent P/D and had epithelioid tumors, median survival was 21 months, whereas it was 11 months for biphasic and sarcomatoid tumors (2). In the following decades technique of P/D was refined to extended P/D with resection of diaphragm and pericardium while sparing the lung (3).
Technical definition
The chaos of surgical terminology in MPM was standardized and refined by International Association for Study of Lung Cancer Thoracic domain and published in 2011 (3).
In this report, EPP was defined as en bloc resection of the parietal and visceral pleura with the ipsilateral lung, pericardium, and diaphragm (Figure 1). According to the group’s recommendation, in cases where the pericardium and/or diaphragm are not involved by tumor, these structures may be left intact. This is well in accordance with the reports from Butchart in 1976 and Sugarbaker in 1999 (1,4). In a retrospective cohort 314 patients who underwent EPP or P/D, there was no evidence of diaphragm involvement in 119 (38%) of the patients (5).
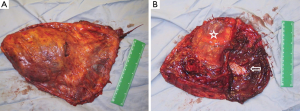
The technique of pleurectomy that involved resection of diaphragm and/or pericardium was defined as radical or extended P/D in several papers and the recommendation was to use the term extended P/D. Extended P/D was defined as parietal and visceral pleurectomy to remove all gross tumor with resection of the diaphragm and/or pericardium. The suggestion was to use the term “extended” rather than “radical”, as the latter implied a completeness of resection with added therapeutic benefit. However, there is currently insufficient evidence that resection of the pericardium and diaphragm provides either.
P/D was defined as performance of parietal and visceral pleurectomy and removal of all gross tumor without removal of diaphragm and pericardium whereas partial P/D was defined as partial removal of parietal and/or visceral pleura for diagnostic or palliative purposes but obviously leaving gross tumor behind.
Comparison of EPP with P/D
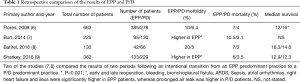
We have several retrospective studies comparing EPP with P/D, but these two techniques have not been compared in a prospective randomized trial. The underpowered randomized trial of MARS, compared EPP with a group of patients who mainly received chemotherapy and other not specified surgical interventions. The four studies that have specifically focused on the comparison of EPP and P/D are shown in Table 1 (6-8).
Full table
Despite the limitations of retrospective studies, these studies show that morbidity and mortality is higher following EPP, while survival figures are similar.
Morbidity
Morbidities related with EPP is much different than P/D. Removal of the lung, hemidiaphragm and ipsilateral pericardium leads to a significant hemodynamic and respiratory challenge and leads to cardiorespiratory complications specific to the procedure. Most common complication following a P/D is prolonged air leak which is related with the trauma to the lung tissue while peeling of the pleura. The morbidities seen in large EPP and P/D series are shown in Table 2.

Full table
Mortality and reasons for mortality
The frequency and causes of mortality are different in EPP and P/D. High risk of bronchopleural fistula in right sided EPP was usually associated with sepsis and subsequent pulmonary and multiorgan failure (11). Pulmonary embolism was also a leading cause of mortality in patients who underwent EPP. In the report by Sugarbaker, deep vein thrombosis was observed in 21/328 patients (6.4%), which resulted in pulmonary embolism in 5 (1.5%) of the patients (13). In most series, P/D patients were older, with higher comorbidities and limited pulmonary function (7). As a result, P/D patients had more atelectasis, pneumonia, prolonged air leak, localized infections, pulmonary embolism and empyema which can lead to mortal situations. Frequency and causes of mortality are listed in Table 3.

Full table
Mortality rate after EPP was significantly high (>10%) in the early years, however in most experienced centers the mortality rate lowered to less than 5% in the last decade (1,13,17). In the study from the database of the Society of Thoracic Surgeons, EPP mortality was 6.5% in high volume (>5/year) centers, whereas it was 12.5% in low volume centers.
P/D is almost always associated with low mortality rate. Lang-Lazdunski reported no mortality at 30 and 90 days in 102 patients (10), however when intraoperative heated chemotherapy was added mortality was 11% (5/44) in a prospective patient series from an experienced center (18). This high mortality rate was attributed to advanced age and limited pulmonary function.
Impact of multimodality treatment and treatment compliance
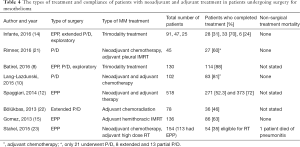
Multimodality treatment is now considered the standard of care in MPM. Various treatment schemes have been used including neoadjuvant, intraoperative and adjuvant treatments. One of the first reports that utilized adjuvant high dose radiation (54 Gy) was the phase II study by Rusch and colleagues (19). The study started with 88 patients. Sixty two underwent EPP, 5 had P/D and 21 had exploratory procedures only. Postoperative mortality was 7.9% (7/88). Adjuvant radiation was administered to 57 patients which showed a very high compliance rate (85%) among resected patients. There is a recent experimental protocol that involves administration of neoadjuvant intensity modulated radiation of 25 Gy in 5 days followed by EPP. The results were very promising in epithelioid tumors with a median survival of 51 months (20). The studies about types of multimodality treatment and treatment compliance are shown in Table 4. The types of treatment are extremely variable between the series, despite one study showing a significant difference for adherence to adjuvant treatment after EPP versus P/D (14), the other studies failed to show any significant difference (8,9).
Full table
Survival outcomes
Prognosis following MPM surgery is still very dismal with most of series reporting two year survival rates less than 40%. The most important prognostic factor is histology with epithelioid disease (4,6,8,10,15), followed by N0 status (8,15,24). Multimodality treatment and female gender were also identified as prognostic factors in a few studies (6,8,12,24). Interestingly only two studies (one P/D and one EPP series) reported macroscopic complete resection to be prognostic factor (4,10). When published data was analyzed, addition of surgery to a multimodality treatment protocol resulted in a survival extension of a maximum of 9 months (25). Long term survival was analyzed in two studies. Eighteen percent of the patients (n=117) survived longer than 3 years after EPP and median age, epithelioid histology and hematologic criteria (normal White blood cell count, hemoglobin and platelets) were found to be significant prognostic factors (26). Another multicentric study on EPP showed that 23% of patients lived longer than 3 years and similarly age and histology were significant prognostic factors followed by no history of asbestos exposure and metastatic/normal lymph node ratio (27). In another study, patients who had stage III MPM and underwent radical pleurectomy followed by chemoradiation, 37% survived longer than 3 years (22). Two-, five-year and median survivals following surgical treatment of MPM are presented in Table 5. In these series, progression free survival was less than one year. As seen in Table 5, the treatment protocols were significantly different between the series, but other than a few series with limited number of patients, median and long term survivals were almost identical between EPP and P/D.

Full table
Recurrence data
Despite the improvements in overall survival over the decades, the recurrence pattern following surgical treatment of MPM has stayed the same. Only high dose hemithoracic irradiation and IMRT has proved to change the recurrence pattern following EPP (15,19). In those patients, locoregional recurrence has decreased dramatically and most of the recurrences occurred distally, in contralateral hemithorax or abdomen. In a phase II study of 62 patients who underwent EPP and high dose hemithoracic radiation, 54 recurred and only in 7 there was locoregional recurrence, whereas recurrence occurred distally in 30 patients (19). In another series of 136 patients who underwent EPP, 86 also had hemithoracic IMRT. Only 2 patients had only locoregional recurrence. Fifty one patients had distant recurrence with contralateral hemithorax being the most common followed by abdomen (15).
In another series, 169 patients underwent EPP (heated intraoperative chemotherapy rate of 78%) and 62% were epithelioid. Recurrences in ipsilateral hemithorax, contralateral hemithorax, abdomen and other sites were exactly the same in their 1997 and 2015 reports (28,29). Around 70% of the patients had locoregional, 50% had abdominal and 35% had contralateral hemithoracic recurrence. Thus local chemotherapy did not lead to any difference in terms of recurrence patterns.
In patients who underwent extended P/D and postoperative chemoradiation, only locoregional recurrence occurred in 47% of the patients, followed by distant and both (14% each) (22). When two practice periods were compared, EPP predominant period had more distant recurrences, while P/D predominant period had more locoregional recurrences (8).
In a novel technique that involves administration of accelerated neoadjuvant hemithoracic IMRT, EPP was performed in 62 patients subsequently. Survival was very good for epithelioid histology patients. Only 30 patients had recurrence and ipsilateral hemithoracic recurrence was seen in 8 patients and these were in patients with biphasic histology and clinical T4N2 disease. Remainder of the recurrences were in the contralateral chest or abdomen (20).
Despite the changes in recurrence patterns, in most of the series this does not translate to any survival advantage between the two techniques.
Final word
The surgical technique in MPM aims to achieve a macroscopic complete resection. The best technique to achieve this was with EPP in the beginning. The technique of EPP, a morbid and risky procedure, has been refined over time and became a procedure with acceptable morbidity and mortality. However, technique of P/D has also improved with diaphragmatic and pericardial resection and acceptable macroscopic complete resection can also be achieved with P/D in the current era. The morbidity and mortality is much less following P/D. In several large patient series of EPP and P/D, there was no difference in terms of long term survival. The main prognostic factors were epithelioid histology, extrapleural lymph node metastasis and completion of multimodality treatment. Based on the current evidence, the choice of a less morbid and mortal procedure (P/D) seems to be the logical choice in the treatment of MPM.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Butchart EG, Ashcroft T, Barnsley WC, et al. Pleuropneumonectomy in the management of diffuse malignant mesothelioma of the pleura. Experience with 29 patients. Thorax 1976;31:15-24. [Crossref] [PubMed]
- Wanebo HJ, Martini N, Melamed MR, et al. Pleural mesothelioma. Cancer 1976;38:2481-8. [Crossref] [PubMed]
- Rice D, Rusch V, Pass H, et al. Recommendations for uniform definitions of surgical techniques for malignant pleural mesothelioma: a consensus report of the international association for the study of lung cancer international staging committee and the international mesothelioma interest group. J Thorac Oncol 2011;6:1304-12. [Crossref] [PubMed]
- Sugarbaker DJ, Flores RM, Jaklitsch MT, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg 1999;117:54-63; discussion 63-5. [Crossref] [PubMed]
- Sharkey AJ, Bilancia R, Tenconi S, et al. The management of the diaphragm during radical surgery for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2016;50:311-6. [Crossref] [PubMed]
- Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg 2008;135:620-6, 626.e1-3.
- Burt BM, Cameron RB, Mollberg NM, et al. Malignant pleural mesothelioma and the Society of Thoracic Surgeons Database: an analysis of surgical morbidity and mortality. J Thorac Cardiovasc Surg 2014;148:30-5. [Crossref] [PubMed]
- Batirel HF, Metintas M, Caglar HB, et al. Adoption of pleurectomy and decortication for malignant mesothelioma leads to similar survival as extrapleural pneumonectomy. J Thorac Cardiovasc Surg 2016;151:478-84. [Crossref] [PubMed]
- Sharkey AJ, Tenconi S, Nakas A, et al. The effects of an intentional transition from extrapleural pneumonectomy to extended pleurectomy/decortication. Eur J Cardiothorac Surg 2016;49:1632-41. [Crossref] [PubMed]
- Lang-Lazdunski L, Bille A, Papa S, et al. Pleurectomy/decortication, hyperthermic pleural lavage with povidone-iodine, prophylactic radiotherapy, and systemic chemotherapy in patients with malignant pleural mesothelioma: a 10-year experience. J Thorac Cardiovasc Surg 2015;149:558-65; discussion 565-6. [Crossref] [PubMed]
- Lauk O, Hoda MA, de Perrot M, et al. Extrapleural pneumonectomy after induction chemotherapy: perioperative outcome in 251 mesothelioma patients from three high-volume institutions. Ann Thorac Surg 2014;98:1748-54. [Crossref] [PubMed]
- Spaggiari L, Marulli G, Bovolato P, et al. Extrapleural pneumonectomy for malignant mesothelioma: an Italian multicenter retrospective study. Ann Thorac Surg 2014;97:1859-65. [Crossref] [PubMed]
- Sugarbaker DJ, Jaklitsch MT, Bueno R, et al. Prevention, early detection, and management of complications after 328 consecutive extrapleural pneumonectomies. J Thorac Cardiovasc Surg 2004;128:138-46. [Crossref] [PubMed]
- Infante M, Morenghi E, Bottoni E, et al. Comorbidity, postoperative morbidity and survival in patients undergoing radical surgery for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2016;50:1077-82. [Crossref] [PubMed]
- Gomez DR, Hong DS, Allen PK, et al. Patterns of failure, toxicity, and survival after extrapleural pneumonectomy and hemithoracic intensity-modulated radiation therapy for malignant pleural mesothelioma. J Thorac Oncol 2013;8:238-45. [Crossref] [PubMed]
- Neragi-Miandoab S, Richards WG, Sugarbaker DJ. Morbidity, mortality, mean survival, and the impact of histology on survival after pleurectomy in 64 patients with malignant pleural mesothelioma. Int J Surg 2008;6:293-7. [Crossref] [PubMed]
- Rusch VW, Piantadosi S, Holmes EC. The role of extrapleural pneumonectomy in malignant pleural mesothelioma. A Lung Cancer Study Group trial. J Thorac Cardiovasc Surg 1991;102:1-9. [PubMed]
- Richards WG, Zellos L, Bueno R, et al. Phase I to II study of pleurectomy/decortication and intraoperative intracavitary hyperthermic cisplatin lavage for mesothelioma. J Clin Oncol 2006;24:1561-7. [Crossref] [PubMed]
- Rusch VW, Rosenzweig K, Venkatraman E, et al. A phase II trial of surgical resection and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2001;122:788-95. [Crossref] [PubMed]
- de Perrot M, Feld R, Leighl NB, et al. Accelerated hemithoracic radiation followed by extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2016;151:468-73. [Crossref] [PubMed]
- Rimner A, Zauderer MG, Gomez DR, et al. Phase II Study of Hemithoracic Intensity-Modulated Pleural Radiation Therapy (IMPRINT) As Part of Lung-Sparing Multimodality Therapy in Patients With Malignant Pleural Mesothelioma. J Clin Oncol 2016;34:2761-8. [Crossref] [PubMed]
- Bölükbas S, Eberlein M, Kudelin N, et al. Factors predicting poor survival after lung-sparing radical pleurectomy of IMIG stage III malignant pleural mesothelioma. Eur J Cardiothorac Surg 2013;44:119-23. [Crossref] [PubMed]
- Stahel RA, Riesterer O, Xyrafas A, et al. Neoadjuvant chemotherapy and extrapleural pneumonectomy of malignant pleural mesothelioma with or without hemithoracic radiotherapy (SAKK 17/04): a randomised, international, multicentre phase 2 trial. Lancet Oncol 2015;16:1651-8. [Crossref] [PubMed]
- Sugarbaker DJ, Richards WG, Bueno R. Extrapleural pneumonectomy in the treatment of epithelioid malignant pleural mesothelioma: novel prognostic implications of combined N1 and N2 nodal involvement based on experience in 529 patients. Ann Surg 2014;260:577-80; discussion 580-2. [Crossref] [PubMed]
- Utley M, Fiorentino F, Treasure T. Obtaining an upper estimate of the survival benefit associated with surgery for mesothelioma. Eur J Cardiothorac Surg 2010;38:241-4. [Crossref] [PubMed]
- Sugarbaker DJ, Wolf AS, Chirieac LR, et al. Clinical and pathological features of three-year survivors of malignant pleural mesothelioma following extrapleural pneumonectomy. Eur J Cardiothorac Surg 2011;40:298-303. [PubMed]
- Leuzzi G, Rea F, Spaggiari L, et al. Prognostic Score of Long-Term Survival After Surgery for Malignant Pleural Mesothelioma: A Multicenter Analysis. Ann Thorac Surg 2015;100:890-7. [Crossref] [PubMed]
- Baldini EH, Recht A, Strauss GM, et al. Patterns of failure after trimodality therapy for malignant pleural mesothelioma. Ann Thorac Surg 1997;63:334-8. [Crossref] [PubMed]
- Baldini EH, Richards WG, Gill RR, et al. Updated patterns of failure after multimodality therapy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2015;149:1374-81. [Crossref] [PubMed]


