Ultrasound capsule endoscopy: sounding out the future
Introduction
The introduction of video capsule endoscopy (VCE) has been a technical boon to the diagnosis and management of gastrointestinal (GI) disorders (1) with the ability to non-invasively image the mucosa of the entire GI tract. This is especially true for the small bowel (SB) which has previously been difficult to image directly. Despite the obvious benefits, VCE suffers from a number of limitations including the inability to biopsy, poor capsule/lesion localization and dependency on gut peristalsis for locomotion (2). In addition to these well recognized impediments is the restriction to analysis of only the superficial mucosa due to VCE’s reliance on visible light for imaging (3).
Imaging limitations
Visible light rays range between 400–700 nm and are strongly attenuated by tissue at depths of 100–1,000 µm (3) with a diminished return of light to the camera. Thus only the mucosal surface can be analyzed and subsurface pathology cannot be imaged and evaluated. Reliance on superficial manifestations of disease opens interpretation to a number of pitfalls regarding lesions that are visually obscure or occult, variable in appearance, patchy in distribution and/or occurring in microfoci (4,5). Furthermore, pathologic mucosal visual changes often cannot be considered specific due to visual overlaps between diseases (6) and sensitivity declines when encountering lower grade diseases, as visible manifestation is less overt (7).
Non-optical capsules
Attempts have been made to develop capsule endoscopes that do not rely on visible light and allow subsurface visualization. C-Scan® Cap (Check-Cap Ltd, ISR), for instance, is being developed as an X-ray based imaging capsule (8). Gora and colleagues have developed an endomicroscopy capsule (9,10). Designed to detect metaplastic and dysplastic changes associated with Barrett’s esophagus, this tethered capsule employs optical coherence tomography to provide high resolution (<10 µm) axial sections of the esophagus.
Ultrasound capsule endoscopy (USCE)
After earlier research with limited outcomes (11,12), USCE is under development in several groups including those led by Khuri-Yakub at Stanford University (USA) (13) and Qiu at Shenzhen (CHN). The largest such activity (Sonopill, UK EPSRC reference GR/K034537/2), is a multi-institutional programme with the ultimate aim to incorporate microultrasound (µUS) and video modalities into a 10 mm diameter by 30 mm long capsule, as depicted in Figure 1. This will allow simultaneous optical mucosal visualization and transmural µUS imaging in a manner similar to conventional endoscopic ultrasound (EUS). However, USCE will have full GI tract transit with a higher µUS spatial resolution with ultrasound (US) imaging limited to the bowel wall. To accomplish this within the volume restrictions of an ingestible capsule, the µUS transducer array and associated electronics must be: microscale reducible, biologically safe and cost effective single-use device. As a means of imaging that is already clinically established, µUS met the above criteria in terms of miniaturization (15,16), safety (i.e., nonionizing radiation) and relatively low manufacturing costs (17).
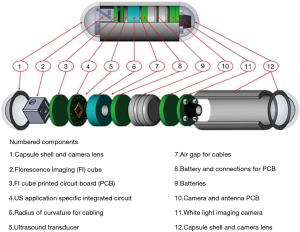
An important aspect of USCE development is to incorporate an imaging modality capable of transmural visualization with higher resolution than conventional EUS. To achieve this, µUS has been considered as the modality of choice. µUS frequencies are more typically a factor at least 1.5 times higher than standard clinical frequencies which generally operate at a maximum of 20 MHz (18). Nevertheless, µUS operates under the same physical principles as conventional clinical US. It is sometimes called ultrasound biomicroscopy (UBM) with reference to potential micrometer axial and lateral resolution. Early results in UBM from Sherar et al. (19) demonstrated it to be capable of non-invasively imaging subsurface structures and tumor spheroids with a 100 MHz US transducer and µUS has consequently become established in clinical applications such as ophthalmology (20), dermatology (21) and intravascular ultrasound (IVUS) (22).
US physics
US waves are produced by the application of electrical voltage to a piezoelectric material such as quartz or piezoceramic. The electrical signal causes a material deformation which generates a high frequency pressure wave. US waves generated by an array of piezoelectric transducers are transmitted through tissue and echoic reflections from tissue interfaces and other features return to the probe. The echoes are detected by the transducer array and are converted to electrical signals which are combined to generate images based on a time-distance relationship.
With µUS, the improved axial and lateral resolution is the result of greater US wave interaction with microscopic tissue components. As US frequency is increased, the wavelength shortens and microstructures normally too small to generate a distinct signal at conventional frequencies become acoustically manifest at higher frequencies. This allows for improved discrimination between adjacent microstructures. The tissue echoes can be reconstructed in a brightness-mode (B-mode) image or other conventional image display formats.
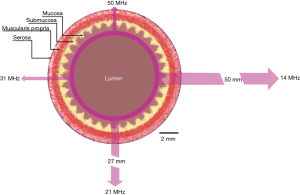
The trade-off for increasing frequency and hence spatial resolution is a decrease in depth of US penetration. Figure 2 illustrates the phenomena of changing resolution and tissue penetration depth as a function of US frequency (23). In general, US attenuation is a result of interactions between the US wave and tissue resulting in signal scattering and absorption. Both are strongly related to frequency and higher frequencies will experience increased signal loss. However, this relationship has the potential to be beneficial for limiting the region of interest (ROI) to the bowel wall itself. Hence, despite the potential for user complaints regarding the lack of penetration (24), this could reduce the amount of superfluous and potentially confounding data gathered as is for example, in transabdominal sonography (TABS) assessment of bowel inflammation (25).
Qualitative aspects
The ability of µUS to characterize GI tissue with a high degree of agreement with histological analysis has been established (26,27). Part of the strong correlation between µUS images and histology stems from the ability of µUS to provide high resolution images, as noted in the previous section. High frequency catheter mini-probes have been developed for upper and lower GI examination in conjunction with standard endoscopy, employing frequencies ranging from below 20 MHz to greater than 30 MHz (28). Primary indications include use for local ‘T’ staging and establishing the feasibility of endoscopic mucosal resection (EMR) (29,30).
Standard EUS frequencies usually generates a five-layer image that correlates with the lumen to gut wall interface and the cardinal transmural layers consisting of the mucosa, submucosa, muscularis propria and serosa. Higher frequency sonography can depict bowel wall structure with additional details and layers (26,27,31). This additional detail makes µUS well suited for imaging the gut wall for subsurface and transmural defects.
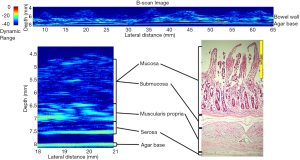
Results using a single element µUS probe have also revealed a high degree of correlation between µUS and SB histology (32). The authors’ work illustrated in Figure 3 demonstrates the degree of correlation between a 47.7 MHz scan of a section of explanted porcine SB and corresponding histology1. The µUS scan demonstrates three distinct layers corresponding to the combined mucosa/submucosa, muscularis propria and serosa as opposed to the four distinct layers of the histology slide consisting of mucosa, submucosa, muscularis propria and serosa. The lack of differentiation between the mucosa and submucosa may be due to insufficient change in the acoustic impedance between these layers in the ex vivo samples.
Existing data suggest that µUS imaging could allow direct imaging of mucosal and/or transmural pathology in a way that is not possible with conventional frequencies. For instance, TABS imaging of coeliac disease and inflammatory bowel disease (IBD) can be achieved with conventional US frequencies (33-35), but the findings of increased luminal fluid, luminal dilation, mural thickening, mesenteric lymphadenopathy and increased peristalsis, are generally nonspecific. Other issues with TABS include assessment that can be hindered by a large body habitus and is a generally non-continuous scan of the GI tract. Furthermore, distinguishing adjacent bowel loops from mural thickening can be difficult (35). Direct bowel imaging using µUS in capsule form has the potential for direct imaging of bowel wall pathology whilst avoiding the shortcomings associated with TABS.
Additionally, the ability to analyze a lesion in situ using µUS and other combined diagnostic modalities has the potential to further develop the concept of in vivo pathology or virtual histology (36,37). As noted earlier, a marked limitation of VCE is its inability to obtain tissue for analysis. This deficiency leads to a requirement for conventional endoscopic or surgical follow-up if a biopsy or intervention is deemed necessary. The ability to characterize a lesion in situ and differentiate between malignant and benign, at a minimum, possibly reduces the need for invasive follow-up.
Frequency choice
As noted earlier, µUS operates at frequencies minimally greater than 20 MHz and typically greater than 30 MHz to achieve improved lateral and axial resolution. This indicates that there is a wide frequency spectrum in which to adopt USCE. The importance of this property relates to the balance between adequate resolution for diagnostic yield and data generated as it relates to interpretation times. The issue of already lengthy reading times has been addressed in the literature (38) and the addition of a second modality to a capsule, such as µUS, has the potential to significantly increase interpretation time. Therefore determination of a frequency that meets the needs for diagnostic accuracy without overburdening the clinician with data is an area of active research (32).
Quantitative aspects
A notable aspect of US imaging is the data acquisition method used to reconstruct an image. Echoes generated by tissue are affected by tissue density and the speed of wave propagation. The qualitative images typical of US are formed from these reflections but this image also contains quantitative information about the physical properties of tissue examined (39). Calculation of the physical or acoustic properties of tissue from the reflected signals is termed quantitative ultrasound (QUS) and this adds objective and measurable parameters to US data (40).
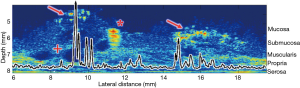
Tissues undergoing pathologic changes have the potential to affect the acoustic properties as demonstrated by Fatehullah et al. (41). This paper concluded that tissue architectural changes could be detected with both qualitative and quantitative US prior to being detectable with conventional histological means. Work in combined biologic/inorganic and organic phantoms using QUS with µUS has been conducted on porcine SB1 (42). Figure 4 illustrates the quantitative and qualitative results of a scan of porcine SB1 infused with hyperechoic microspheres at an US frequency of 47.7 MHz with graphic overlay indicating quantitative changes in MRayl attenuation. As the scan passes from unperfused tissue to regions containing microsphere aggregates there is a noticeable change in signal attenuation. In this analysis, only the first 100 µm depth of the sample was analyzed quantitatively for changes. Data from below that depth were ignored which included a polystyrene fiducial marker. The ability to choose the tissue depth to be analyzed allows focusing on a ROI restricted to layers of user interest.
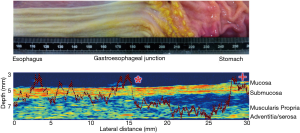
Further work is under way to measure qualitative and quantitative mucosal changes in porcine esophagus to detect transition from the stratified squamous to simple columnar mucosal lining at the gastroesophageal junction. Figure 5 shows a full thickness scan again at 47.7 MHz for an explanted porcine esophagus as it transitions into the stomach1. The overlaid graph of MRayl attenuation changes as the scan progresses from proximal to distal at the area of the gastroesophageal junction.
Computer assisted diagnosis
A major advantage of the quantitative aspect of µUS is its potential to be adapted to computer assisted diagnosis (CADx). Means of reducing the time commitment to review the clinical data is ranked high on the clinician’s ‘wish list’ of VCE improvement due to the lengthy interpretation times currently experienced (43). QUS-based image analysis has already been studied in IVUS (44) where Timmins et al. demonstrated quantifiable changes in coronary atherosclerotic plaques and postulated that automated quantitative methods could improve and accelerate lesion analysis. The previously discussed ROI control and the quantitative factors of µUS demonstrate promise for adapting QUS to an automated interpretation process. An automated QUS diagnostic method based on the acoustic property differences between healthy and diseased GI tissue could be developed where abnormal quantitative results can possibly be used to direct physician attention to particular areas of concern.
Conclusions
Efforts are under way to develop USCE with the inclusion of µUS to allow for high resolution transmural imaging of the gut wall. Given the close proximity of the US probe and the relevant tissue, µUS could be able to provide direct evidence of subsurface and transmural pathology. This will overcome the issues associated with white light imaging, where there is a reliance on visual disease manifestation. It will also address issues associated with TABS, in regards to relying less on nonspecific signs of inflammation, and overcome problems associated with transcutaneous US. Furthermore, µUS can be potentially adapted to automated diagnosis by applying the quantitative aspects of US. By ascertaining the acoustic properties of tissue, data can be presented in an objective and measurable way.
Laboratory experiments continue with investigations into various aspects of capsule development. This includes a study to determine which µUS frequency provides optimal diagnostic yield. Other work is also considering the development of the electronic hardware and software necessary for the capsule functionality. This includes microchip design, µUS array development, integration of the functional sub-elements and capsule shell functionalization. While conceived primarily as an US capable diagnostic capsule, other sensing modalities are under consideration, including fluorescent imaging (14). Development of therapeutic capsules is another area of active research (45).
Current research efforts have entered the translational phase with large animal trials being conducted at the Roslin Institute, University of Edinburgh2. Tethered versions of single modality capsules are being tested in the upper GI tract and SB of anaesthetized pigs (results not shown). These trials are designed to address fundamental questions regarding the USCE development. Chief amongst the investigations is to determine if there is adequate coupling between USCE transducer array and mucosa to facilitate US imaging. Additional experiments have examined the thermogenic profile of an USCE device to aid in power budgeting. Translational trials will continue with further refinement of USCE and also test other diagnostic and therapeutic modalities.
Acknowledgements
The authors wish to thank Dr. Vipin Seetohul for the schematic drawing of Sonopill and Robyn Duncan for the histology slide.
Funding: This work was supported by the UK Engineering and Physical Sciences Research Council (EPSRC) Sonopill grant (EP/K034537/2).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
1 Laboratory work was conducted on porcine small bowel and esophageal samples that were abattoir obtained (Medical Meats Supplies, Oldham UK).
2 This study was approved by the Animal Welfare and Ethical Review Board of the Roslin Institute, Roslin, Midlothian, EH25 9RG and is being carried out under Home Office (UK) License PPL 7008812.
References
- Koulaouzidis A, Rondonotti E, Karargyris A. Small-bowel capsule endoscopy: a ten-point contemporary review. World J Gastroenterol 2013;19:3726-46. [Crossref] [PubMed]
- Koprowski R. Overview of technical solutions and assessment of clinical usefulness of capsule endoscopy. Biomed Eng Online 2015;14:111. [Crossref] [PubMed]
- Nakamura K. Further Development of Endoscopic Imaging: “Era of Light” Activities with Optics and Image Processing Technology. In: Niwa H, Tajiri H, Nakajima M, et al. editors. New Challenges in Gastrointestinal Endoscopy. Tokyo: Springer Japan, 2008:40-54.
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007;448:427-34. [Crossref] [PubMed]
- Ciaccio EJ, Bhagat G, Lewis SK, et al. Suggestions for automatic quantitation of endoscopic image analysis to improve detection of small intestinal pathology in celiac disease patients. Comput Biol Med 2015;65:364-8. [Crossref] [PubMed]
- Dickson BC, Streutker CJ, Chetty R. Coeliac disease: an update for pathologists. J Clin Pathol 2006;59:1008-16. [Crossref] [PubMed]
- Kav T, Sivri B. Is enteroscopy necessary for diagnosis of celiac disease? World J Gastroenterol 2012;18:4095-101. [Crossref] [PubMed]
- Gluck N, Shpak B, Brun R, et al. A novel prepless X-ray imaging capsule for colon cancer screening. Gut 2016;65:371-3. [Crossref] [PubMed]
- Gora MJ, Sauk JS, Carruth RW, et al. Imaging the Upper Gastrointestinal Tract in Unsedated Patients Using Tethered Capsule Endomicroscopy. Gastroenterology 2013;145:723-5. [Crossref] [PubMed]
- Gora MJ, Sauk JS, Carruth RW, et al. Tethered capsule endomicroscopy enables less invasive imaging of gastrointestinal tract microstructure. Nat Med 2013;19:238-40. [Crossref] [PubMed]
- Final Report Summary - TROY (Endoscope Capsule using Ultrasound Technology). Available online: http://cordis.europa.eu/documents/documentlibrary/125822911EN6.pdf. Accessed Mar 23, 2017.
- Lee JH, Traverso G, Schoellhammer CM, et al. Towards wireless capsule endoscopic ultrasound (WCEU). 2014 IEEE International Ultrasonics Symposium. IEEE, 2014:734-7.
- Memon F, Touma G, Wang J, et al. Capsule ultrasound device: Further developments. 2016 IEEE International Ultrasonics Symposium (IUS). IEEE, 2016:1-4.
- Al-Rawhani MA, Beeley J, Cumming DR. Wireless fluorescence capsule for endoscopy using single photon-based detection. Sci Rep 2015;5:18591. [Crossref] [PubMed]
- Szabo TL. Diagnostic ultrasound imaging: inside out. In: Joseph Bronzino SE, editor. Academic press series in biomedical engineering. Burlington: Elsevier, 1991:iii.
- Triger S, Saillant JF, Demore CE, et al. Low-voltage coded excitation utilizing a miniaturized integrated ultrasound system employing piezoelectric 2-D arrays. IEEE Trans Ultrason Ferroelectr Freq Control 2010;57:353-62. [Crossref] [PubMed]
- Nakamura K. Ultrasonic Transducers. In: Ultrasonic Transducers. Elsevier, 2012.
- Vogt M, Opretzka J, Perrey C, et al. Ultrasonic microscanning. Proc Inst Mech Eng H 2010;224:225-40. [Crossref] [PubMed]
- Sherar MD, Noss MB, Foster FS. Ultrasound backscatter microscopy images the internal structure of living tumour spheroids. Nature 1987;330:493-5. [Crossref] [PubMed]
- Silverman RH. High-resolution ultrasound imaging of the eye - A review. Clin Exp Ophthalmol 2009;37:54-67. [Crossref] [PubMed]
- Dasgeb B, Kainerstorfer J, Mehregan D, et al. An introduction to primary skin imaging. Int J Dermatol 2013;52:1319-30. [Crossref] [PubMed]
- McKay CR, Shavelle DM. Intravascular ultrasound in the coronary arteries. Semin Vasc Surg 2006;19:132-8. [Crossref] [PubMed]
- Fried NM, Burnett AL. Novel methods for mapping the cavernous nerves during radical prostatectomy. Nat Rev Urol 2015;12:451-60. [Crossref] [PubMed]
- Bhutani MS. “Probing” the endoscopic ultrasound (EUS) catheter probe: a small step for EUS or a giant leap? Gastrointest Endosc 1998;48:542-5. [Crossref] [PubMed]
- Nylund K. Sonography of the small intestine. World J Gastroenterol 2009;15:1319. [Crossref] [PubMed]
- Wiersema MJ, Wiersema LM. ultrasonography of the gastrointestinal wall : histologic correlates. Gastrointest Endosc 1993;39:499-504. [Crossref] [PubMed]
- Ødegaard S, Nesje LB, Lærum OD, et al. High-frequency ultrasonographic imaging of the gastrointestinal wall. Expert Rev Med Devices 2012;9:263-73. [Crossref] [PubMed]
- Schembre D, Ayub K, Jiranek G. High-frequency mini-probe ultrasound: the Rodney Dangerfield of endoscopy? J Clin Gastroenterol 2005;39:555-6. [Crossref] [PubMed]
- Khanna LG, Gress FG. Preoperative evaluation of oesophageal adenocarcinoma. Best Pract Res Clin Gastroenterol 2015;29:179-91. [Crossref] [PubMed]
- Gall TM, Markar SR, Jackson D, et al. Mini-probe ultrasonography for the staging of colon cancer: a systematic review and meta-analysis. Colorectal Dis 2014;16:O1-8. [Crossref] [PubMed]
- Ødegaard S. High-resolution endoluminal sonography in gastroenterology. Eur J Ultrasound 1999;10:85-91. [Crossref] [PubMed]
- Anbarasan T, Démoré CE, Lay H, et al. High Resolution Microultrasound (µUS) Investigation of the Gastrointestinal (GI) Tract. Methods Mol Biol 2017;1572:541-61. [Crossref] [PubMed]
- Fraquelli M, Colli A, Colucci A, et al. Accuracy of ultrasonography in predicting celiac disease. Arch Intern Med 2004;164:169-74. [Crossref] [PubMed]
- Bartusek D, Valek V, Husty J, et al. Small bowel ultrasound in patients with celiac disease. Retrospective study. Eur J Radiol 2007;63:302-6. [Crossref] [PubMed]
- Strobel D, Goertz RS, Bernatik T. Diagnostics in inflammatory bowel disease: ultrasound. World J Gastroenterol 2011;17:3192-7. [PubMed]
- Carignan CS, Yagi Y. Optical endomicroscopy and the road to real-time, in vivo pathology: present and future. Diagn Pathol 2012;7:98. [Crossref] [PubMed]
- Layland J, Wilson AM, Lim I, et al. Virtual Histology: A Window to the Heart of Atherosclerosis. Heart Lung Circ 2011;20:615-21. [Crossref] [PubMed]
- Lo SK. How Should We Do Capsule Reading? Tech Gastrointest Endosc 2006;8:146-8. [Crossref]
- Lieu D. Ultrasound physics and instrumentation for pathologists. Arch Pathol Lab Med 2010;134:1541-56. [PubMed]
- Mamou J, Oelze M. editors. Quantitative Ultrasound in Soft Tissues. Dordrecht: Springer Netherland, 2013.
- Fatehullah A, Sharma S, Newton IP, et al. Increased variability in ApcMin/+ intestinal tissue can be measured with microultrasound. Sci Rep 2016;6:29570. [Crossref] [PubMed]
- Lay HS, Cox BF, Sunoqrot M, et al. Microultrasound characterisation of ex vivo porcine tissue for ultrasound capsule endoscopy. J Phys Conf Ser 2017;797:12003. [Crossref]
- Fireman Z. Capsule endoscopy: Future horizons. World J Gastrointest Endosc 2010;2:305-7. [PubMed]
- Timmins LH, Molony DS, Eshtehardi P, et al. Quantification of the focal progression of coronary atherosclerosis through automated co-registration of virtual histology-intravascular ultrasound imaging data. Int J Cardiovasc Imaging 2017;33:13-24. [Crossref] [PubMed]
- Stewart F, Cox B, Vorstius J, et al. Capsule-based ultrasound-mediated targeted gastrointestinal drug delivery. 2015 IEEE International Ultrasonics Symposium (IUS). IEEE, 2015:1-4.


