Microscopic colitis—microbiome, barrier function and associated diseases
Introduction
Microscopic colitis (MC) is a chronic inflammatory bowel disease (IBD) with few or no endoscopic abnormalities (1-3). Patients with MC present chronic, non-bloody watery diarrhoea, however some may suffer from constipation, abdominal pain or even remain symptom free (4,5). Patients with MC are more often middle aged or older women in their seventh decade of life. According to several reports (6) there is steady increase in MC incidence, currently around 10 cases per 100,000 person years, almost comparable to the incidence of other IBDs, such as ulcerative colitis (UC) or Crohn’s disease (CD) (7,8). Of note, MC has been regarded as a subgroup within IBD (8).
The two main subtypes of MC are lymphocytic colitis (LC) and collagenous colitis (CC) (9). Epithelial damage and inflammation in the lamina propria, mainly with mononuclear cells, are observed in both diseases. In LC, the intraepithelial lymphocyte (IEL) count is ≥20/100 epithelial cells and the sub-epithelial collagen layer <10 µm thick. In CC, the IEL count may be increased, but a thickening of the sub-epithelial collagen layer ≥10 µm is necessary for the diagnosis. It has been suggested that both subtypes could be considered as histological subtypes of the same disease, and that in clinical trials, all MC patients could be included (10). Most recently, the type of MC incomplete (MCi) with histopathological changes that are not fulfilling the classic MC criteria has also been described in patients with chronic diarrhoea and normal or close to normal endoscopic findings (1,11). The differential diagnosis between the complete and incomplete MC types remains the pathologist’s challanege and impacts on decision making in clinical practice (12).
MC has significant impact on the health-related quality of life of the patients affected (13). The main aim of medical therapy is to improve the quality of life and achieve clinical remission. In patients with recurrent disease, MC recurrence prevention is desirable. The recommended primary treatment of MC is with the glucocorticoid budesonide (9,14). Antidiarrhoeals or colestyramine only can be considered in MC patients with milder symptoms. In case of relapse, budesonide can be used again either as intermittent or as low-dose continuous therapy. In patients with mild symptoms who do not respond to budesonide, alterative drugs are recommended, such as colestyramine, aminosalicylates or bismuth (9). In some patients MC could be triggered by the use of certain medications, in particular non-steroidal anti-inflammatory drugs (NSAIDs), aspirin, proton pump inhibitors (PPIs), anti-diabetic drugs, and/or antidepressants (15-17). In these cases discontinuation of the medication is the easiest; however not always sufficient solution to treat the MC. Although the primary response to budesonide is often good, with response rate of around 80% (14), relapses occur often (60–80%) when the treatment is stopped (9,18). Besides the relapses, patients in remission can still suffer from persisting symptoms such as abdominal pain, fatigue, arthralgia or myalgia several years after diagnosis (9). The use of anti-TNF-alpha monoclonal antibodies (MoAb) (infliximab, adalimumab) or biosimilars is reserved for the induction of remission in severe cases of MC that fail to respond to corticosteroids or immunomodulators, as an alternative to colectomy (12).
MC has been linked to mucosal inflammation, autoimmune diseases and gut microbiota composition, all of which are also linked to gut barrier function. In this review we would like to summarize the current research to MC in these fields, and to explore the possible roles gut barrier function or microbiota might play in the occurrence and/or treatment of MC.
Etiopathology of MC
The pathophysiological cause of MC is unknown, but recent hypotheses revolve around mucosal inflammation, microbiome and gut barrier alterations. Several extrinsic (environmental) as well as intrinsic (endocrine) factors might play a role in the pathogenesis of the disease.
Epithelial stress and mucosal inflammation
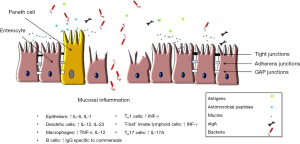
Multiple studies have investigated different mechanisms that might be involved in the development of mucosal inflammation in MC. An increase in the number of T-IELs suggests a specific mucosal immune response to luminal factors in predisposed individuals. Infiltration of CD8+ T lymphocytes has been found in the epithelium of both LC and CC patients, whereas the amount of CD4+ T cells seems to be reduced in the epithelium (19). In the lamina propria, most T lymphocytes seem to be CD4+ (20). Both CD4 and CD8 T cells of MC patients seem to be less active compared to controls (21). Eosinophils also play a role in MC. Increased levels of luminal levels of eosinophilic cationic protein (CP) and an increased number of activated eosinophils in the colonic mucosa have been found in patients with CC (21,22).
Biopsies of sigmoid colon from patients with CC, incubated for 48 h in Roswell Park Memorial Institute (RPMI) medium, produced increased levels of IFN-γ, TNA-α and IL-1 compared to controls (23). Levels of IL-8 and IL-13 did not differ between the groups. Other studies have confirmed the presence of a pro-inflammatory cytokine profiles in biopsies of MC patients (Figure 1) (24-26). Although these studies give an idea of the different mechanisms that may be involved in the development of the mucosal inflammation in MC, the pathophysiological role of these findings is still unclear.
MC and barrier dysfunction
The gut barrier is a complex, multicomponent system in which different cell types in and around the gut wall work together to prevent unwanted translocation of components from the lumen of the gut into the body. Perturbations in the gut barrier function can be due to poor nutrition, infection or other illnesses. This can lead to increased intestinal permeability, the rate of flux of molecules across the epithelium (27). Increased intestinal permeability is associated with a variety of gastrointestinal (GI) disorders, including IBD, irritable bowel syndrome and celiac disease (28). Mucosal barrier dysfunction has also been described in IBD and MC patients (29). Table 1 summerizes studied genes associated with gut barrier alterations in IBD and relevant to MC. This is not surprisingly, since inflammation per se already influences intestinal permeability (43). In CC patients, Ussing chamber experiments with endoscopic biopsies from the sigmoid colon showed that the trans-epithelial resistance was increased during active disease compared to remission and controls (23,44). However, the uptake of chemically killed Escherichia coli K12 in the same model was increased during both active disease and remission (44), as compared with controls, suggesting an underlying barrier dysfunction as cause of frequent and rapid relapses in CC. Small bowel permeability, as measured in vivo with 14C-labelled mannitol and 99mTc-labelled diethylenetriamine-pentaacetic acid seemed to be not altered in CC patients compared with controls (45).

Full table
Budesonide seems to affect the mucosal barrier function, it has anti-proliferative effects on the epithelial cells, which might impair wound healing (46). During significant mucosal injury, it dampens the immune response, causing bacterial translocation and endotoxemia. In a group of LC patients treated with budesonide for at least 4 weeks, no increase in the trans-epithelial resistance was found in Ussing chamber experiments with endoscopic biopsies compared to biopsies of active disease patients (23). Among other factors related to MC incidence are sex hormones (estrogens and progesterone), which have been implicated in anti-inflammatory and epithelial barrier-enhancing properties in animal models of colitis (47,48). The hormonal shifts at the time of menopause could serve as an explanation of the MC increased prevalence among middle-aged women (49).
Numerous video capsule endoscopy (VCE) studies reported that various doses of NSAIDs were responsible for gut mucosal injury with the prevalence ranging from 30% to 80% (50). It has been reported that NSAIDs therapy alters the intestinal barrier function and leads to increased intestinal permeability (51). NSAIDs therapy has been associated with MC; however direct relationship as well as cause and effect of such treatment lacks clinical validation. In fact, some recent reports are against such associations. Zagorowicz et al. (52) found subtle physiological and histopathological differences between the bowel segments in chronic low-dose aspirin users but observed no specific MC features in asymptomatic individuals. It is tempting to speculate that NSAIDs trigger MC through alterations in gut microbiota (52,53) This hypothesis is strengthen by the reports of frequent use of PPIs among CC patients (13). PPIs are frequently combined with NSAIDs and such long-term co-therapy could augment the toxic effect of NSAIDs by induction of dysbiosis. The use of PPIs has been considered as an independent risk factor for small bowel injury in patients with various autoimmune pathologies [e.g., rheumatoid arthritis (54,55)]. The effect of PPIs therapy on GI microbiota composition and function has recently gained scientific attention. PPIs alter various taxa in different regions of GI tract (51,56-59). The hypothesis that gut microbiota play role in the pathogenesis of MC is interesting, however poorly studied yet. Below we discuss the current knowledge concerning the role of human microbiome in MC based on available literature.
MC and microbiome
The gut microbiota play an important role in influencing epithelial barrier functions through the production of short chain fatty acids and interactions with innate immune system, including pattern recognition receptors in the mucosa, driving the expression of mucus and antimicrobial factors (27). In a patient with UC, new onset MC developed after fecal microbiota transplantation (60). This also happened in 2/146 patients who received fecal microbiota transplantation to treat recurrent Clostridium difficile infection (61). In a case series with two patients with CC, the presence of potentially pathogenic Bacteroides spp. were found in biopsies from the ascending colon, but overall the colon microbiota showed similarities to a healthy one (62). In a group of ten patients with onset of MC, decreased levels of Akkermansia muciniphila were found in the fecal samples (63), although this finding might be a consequence of the diarrhoea, and not specific of the MC (64,65). Of interest, Sapp et al. (66) studied the involvement of the distal small intestine in patients with LC and CC. The authors compared the results of those with MC with the results of intestinal biopsies obtained from patients with IBD (CD and UC) and healthy individuals without colonic pathology concluding that intraepithelial lymphocytosis is present in the terminal ileum of patients with LC or CC and may be helpful in distinguishing LC or CC from other forms of IBD in challenging cases. Moreover this study revealed that the terminal ileum may be involved by a similar pathogenic process as the colon in MC. Of note none of the patients in Sapp et al. (66) study had celiac disease—the observation, which makes the scenario that gut microbes are involved in MC pathogenesis interesting. Caminero et al. (67) showed that intestinal bacteria affect mucosal immunogenicity. It is tempting to speculate that other factors (e.g., NSAIDs, smoking or stress)—factors frequently associated with microbiome alterations in the gut—contribute to MC pathology (63). Overall, these are all small studies which might give a clue about a possible role of the microbiota in MC, but much larger studies are necessary to unravel the role of the gut microbiota in MC.
The emerging role of high-definition and super-magnifying endoscopy
The endoscopic and histopathologic criteria for the diagnosis of MC were developed in an era of low-definition endoscopic imaging. The subtle mucosal changes characteristic for MC at that time were simply not observed. New high-definition endoscopic equipment with zoom adjustable high resolution sharp images enable a new vision of colonic mucosa in MC suspected patients. Also other new endoscopic devices with super-magnifying vision in the future might have found their place in MC diagnosis: (I) confocal laser endomicroscopy (CLE; Mauna Kea Technologies, Paris, France; and Pentax, Tokyo, Japan) and (II) an endocytoscopy system (ECS; Olympus, Tokyo, Japan). These endoscopes are unique as they generate the picture, which allows in vivo microscopic observation of the microstructural mucosa of the GI tract (68,69). The new endoscopic devices might be of particular use in MC diagnosis and management by enabling detection of mucosal enteric infections. For example ECS system was much more efficacious in comparison to serologic or histopathologic diagnostic tests in confirming amoebic colitis in in vivo examinations (70). Super-magnifying endoscopes open up new avenues for in vivo identification of microbial enteric infections. These infections are difficult to measure in contemporary clinical practice, but their presence has been strongly postulated to trigger post-infectious alterations of GI mucosa.
MC and association with other diseases
MC patients have a higher prevalence of certain diseases, including a number of autoimmune pathology (8,10,71,72). The diseases most commonly found to coexist with MC are bile acid diarrhoea and autoimmune diseases such as celiac disease, thyroid disease, diabetes mellitus (DM) and rheumatoid arthritis (10). In a recent study with 547 MC patients, increased prevalence of thyroid diseases, rheumatoid arthritis, Raynaud/Crest syndrome, celiac disease and IBS was found compared to a control group (73).
Bile acid diarrhoea is caused by excess bile acid concentration entering the colon. It was initially identified in patients who had undergone resection of the terminal ileum and in patients with ileal CD (27,74). The biochemical structure of certain bile acids has been shown to induce fluid secretion, increase mucosal permeability and produce mucosal damage (46). One study found that 44% of the patients with CC had bile acid diarrhoea (75). Patients with both MC and bile acid diarrhoea usually respond well to bile acid binding treatment (76).
In a cohort of celiac disease patients, 5% of the individuals also had MC, which is higher than in the general population (77). The other way around, in a Swedish cohort of 795 patients with MC, celiac disease occurred in 6% of the patients, whereas the overall occurrence of celiac disease in Sweden is around 3% (78). Celiac disease is an autoimmune disorder primarily affecting the small intestine. The disease is characterized by a strong immunological reaction to gluten. In contrast to MC it has a strong genetic component. The only known effective treatment is a lifelong diet free of gluten.
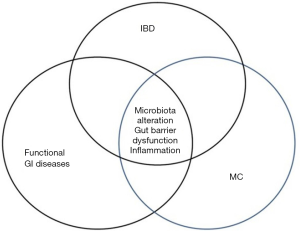
Some of the diseases associated with MC, like bile acid diarrhoea, celiac disease, IBS and DM, are associated with an impaired intestinal barrier function and it has been suggested that impaired barrier function plays a role in the development of autoimmune diseases (79-82). The graphic simplified scheme of GI disease overlap and pertaining to gut barrier alterations has been presented in Figure 2. It is therefore tempting to initiate new clinical studies investigating the role of barrier function in patients with MC and other autoimmune diseases.
Novel therapeutic insights in MC—the role for microbiota management?
Modulation of gut microbiota is an attractive clinical scenario of eliminating gut injury associated with mucosal inflammation. Scarpignato et al. (83) in their recent clinical trial delivered the evidence that intestinal bacteria contribute to the development of NSAIDs-associated enteropathy in humans. In their study, the use of Rifaximine, a non-absorbable antibiotic, contributed to less advanced mucosal lesions in patients treated with NSAIDs.
Another way to interact with the microbiota is by giving probiotics. Probiotics are defined as: live microorganisms that, when administered in adequate amounts, confer a health benefit on the host (84). They have been found to be beneficial in patients with UC or IBS (85), but not so much in patients with CD (86,87). Even when the total microbiota composition is not changed so much by giving probiotics (88), the use of probiotics still has potential for MC, due to changes in microbiota activity, promoting gut microbiota homeostasis and/or immunomodulatory properties (89,90). Another rationale to test probiotics in MC patients, is the protective effects they can have on the epithelial barrier function (91). In pouchitis patients, the intake of a multispecies probiotic (Ecologic825) significantly reduced the passage of E. coli in Ussing chamber experiments (92). In the same model increased E. coli passage was found for CC patients, as mentioned before (44). So it would be interesting to see whether the probiotic has the same effects in MC patients compared with colitis patients.
Some research has been conducted with probiotics in patients with MC. A first open-label trial was done in Germany in 14 patients with CC with the probiotic strain Escherichia coli Nissle 1917 (93). This study found a reduction of the stool frequency and the stool consistency. A double-blinded, placebo controlled trial was done in Denmark in 29 patients with CC with a combination of the bacterial strains Lactobacillus acidophilus LA-5 and Bifidobacterium animalis supsp. lactis BB/12 (94). No significant clinical response to the probiotic intervention was found in this small study, but a trend toward increased stool consistency was found. This was supported by a post hoc analysis showing significant changes in the probiotic group with regards to bowel frequency and faecal consistency (94). An open labelled study in 30 patients from India, with the probiotic mixture VSL#3, suggested that the probiotic could induce short-term clinical response and improve associated symptoms, but no histological response (95). Overall, there is limited research done in patients with MC with probiotics and properly powered trials are required to be able to draw any conclusions. Before starting a large double-blinded, placebo controlled trial, pilots experiments might be necessary to find the most promising patient group, treatment duration and probiotic strains. It is important to keep in mind that properties of probiotic bacteria can be highly variable between strains (96,97), so a good selection of strains is necessary.
Conclusions
Since the first description of MC is 1982, it has become clear that the condition occurs quite often, predominantly in women older than 60 years. Smoking and the use of certain medicine are risk factors to develop the disease. New endoscopic techniques delivering high definition and high-resolution images capable of enhanced visualisation of GI mucosa as well as super magnifying endoscopy (e.g., confocal laser endomicroscopy) open up new avenues in the diagnosis and management of not so evident up to now clinical entities. Modulation of gut microbiota is an attractive therapeutic solution for MC patients. The intestinal barrier function is impaired in MC patients, and this is not restored during budesonide use. This might be a cause of the high relapse rate seen for this disease. It can be speculated that the use of probiotics or other agents capable of modulation of gut microbiota and intestinal barrier might benefit MC patients, but the current evidence is very limited and further clinical studies are necessary to see if probiotics or other new pharmacologic molecules can play a role in the treatment of prevention of relapse of the disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: S van Hemert is employee in Winclove (Winclove manufactures and markets probiotics). I Loniewski and W Marlicz are shareholders in Sanprobi (Sanprobi is a probiotic producing and marketing company). The content of this study was neither influenced nor constrained by these facts. The other authors have no conflicts of interest to declare.
References
- Koulaouzidis A. Distinct colonoscopy findings of microscopic colitis: Not so microscopic after all? World J Gastroenterol 2011;17:4157-65. [Crossref] [PubMed]
- Yung DE, Koulaouzidis A, Fineron P, et al. Microscopic colitis: a misnomer for a clearly defined entity? Endoscopy 2015;47:754-7. [Crossref] [PubMed]
- Koulaouzidis A. Mucosal scars in collagenous colitis. Gastrointest Endosc 2010;71:221-2. [Crossref] [PubMed]
- Ohlsson B. New insights and challenges in microscopic colitis. Therap Adv Gastroenterol 2015;8:37-47. [Crossref] [PubMed]
- Cotter TG, Binder M, Loftus EV Jr, et al. Development of a Microscopic Colitis Disease Activity Index: a prospective cohort study. Gut 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Pardi DS. Diagnosis and Management of Microscopic Colitis. Am J Gastroenterol 2017;112:78-85. [Crossref] [PubMed]
- Tong J, Zheng Q, Zhang C, et al. Incidence, prevalence, and temporal trends of microscopic colitis: a systematic review and meta-analysis. Am J Gastroenterol 2015;110:265-76. [Crossref] [PubMed]
- Fumery M, Kohut M, Gower-Rousseau C, et al. Incidence, Clinical Presentation, and Associated Factors of Microscopic Colitis in Northern France: A Population-Based Study. Dig Dis Sci 2017;62:1571-9. [Crossref] [PubMed]
- Münch A, Aust D, Bohr J, et al. Microscopic colitis: Current status, present and future challenges: statements of the European Microscopic Colitis Group. J Crohns Colitis 2012;6:932-45. [Crossref] [PubMed]
- Rasmussen MA, Munck LK. Systematic review: are lymphocytic colitis and collagenous colitis two subtypes of the same disease - microscopic colitis? Aliment Pharmacol Ther 2012;36:79-90. [Crossref] [PubMed]
- Guagnozzi D, Landolfi S, Vicario M. Towards a new paradigm of microscopic colitis: Incomplete and variant forms. World J Gastroenterol 2016;22:8459-71. [Crossref] [PubMed]
- Fernández-Bañares F, Casanova MJ, Arguedas Y, et al. Current concepts on microscopic colitis: evidence-based statements and recommendations of the Spanish Microscopic Colitis Group. Aliment Pharmacol Ther 2016;43:400-26. [Crossref] [PubMed]
- Hjortswang H, Tysk C, Bohr J, et al. Health-related quality of life is impaired in active collagenous colitis. Dig Liver Dis 2011;43:102-9. [Crossref] [PubMed]
- Chande N, McDonald JW, Macdonald JK. Interventions for treating collagenous colitis. Cochrane Database Syst Rev 2008.CD003575. [PubMed]
- Riddell RH, Tanaka M, Mazzoleni G. Non-steroidal anti-inflammatory drugs as a possible cause of collagenous colitis: a case-control study. Gut 1992;33:683-6. [Crossref] [PubMed]
- Beaugerie L, Pardi DS. Review article: drug-induced microscopic colitis - proposal for a scoring system and review of the literature. Aliment Pharmacol Ther 2005;22:277-84. [Crossref] [PubMed]
- Park T, Cave D, Marshall C. Microscopic colitis: A review of etiology, treatment and refractory disease. World J Gastroenterol 2015;21:8804-10. [Crossref] [PubMed]
- Bouma G, Münch A. Microscopic colitis. Dig Dis 2015;33:208-14. [Crossref] [PubMed]
- Bohr J, Wickbom A, Hegedus A, et al. Diagnosis and management of microscopic colitis: current perspectives. Clin Exp Gastroenterol 2014;7:273-84. [PubMed]
- Mosnier JF, Larvol L, Barge J, et al. Lymphocytic and collagenous colitis: an immunohistochemical study. Am J Gastroenterol 1996;91:709-13. [PubMed]
- Wagner M, Lampinen M, Sangfelt P, et al. Budesonide treatment of patients with collagenous colitis restores normal eosinophil and T-cell activity in the colon. Inflamm Bowel Dis 2010;16:1118-26. [Crossref] [PubMed]
- Taha Y, Carlson M, Thorn M, et al. Evidence of local eosinophil activation and altered mucosal permeability in collagenous colitis. Dig Dis Sci 2001;46:888-97. [Crossref] [PubMed]
- Barmeyer C, Erko I, Fromm A, et al. Ion transport and barrier function are disturbed in microscopic colitis. Ann N Y Acad Sci 2012;1258:143-8. [Crossref] [PubMed]
- Brown EM, Sadarangani M, Finlay BB. The role of the immune system in governing host-microbe interactions in the intestine. Nat Immunol 2013;14:660-7. [Crossref] [PubMed]
- Dey I, Beck PL, Chadee K. Lymphocytic colitis is associated with increased pro-inflammatory cytokine profile and up regulation of prostaglandin receptor EP4. PLoS One 2013;8:e61891. [Crossref] [PubMed]
- Tagkalidis PP, Gibson PR, Bhathal PS. Microscopic colitis demonstrates a T helper cell type 1 mucosal cytokine profile. J Clin Pathol 2007;60:382-7. [Crossref] [PubMed]
- Wells JM, Brummer RJ, Derrien M, et al. Homeostasis of the Gut Barrier and Potential Biomarkers. Am J Physiol Gastrointest Liver Physiol 2017;312:G171-G193. [Crossref] [PubMed]
- Marchiando AM, Graham WV, Turner JR. Epithelial Barriers in Homeostasis and Disease. Annu Rev Pathol 2010;5:119-44. [Crossref] [PubMed]
- Bonagura GA, Ribaldone DG, Fagoonee S, et al. Microscopic colitis in patients with mild duodenal damage: A new clinical and pathological entity (“lymphocytic enterocolitis”)? World J Gastrointest Pathophysiol 2016;7:307-13. [Crossref] [PubMed]
- Ahn SH, Shah YM, Inoue J, et al. Hepatocyte nuclear factor 4alpha in the intestinal epithelial cells protects against inflammatory bowel disease. Inflamm Bowel Dis 2008;14:908-20. [Crossref] [PubMed]
- van Sommeren S, Visschedijk MC, Festen EA, et al. HNF4alpha and CDH1 are associated with ulcerative colitis in a Dutch cohort. Inflamm Bowel Dis 2011;17:1714-8. [Crossref] [PubMed]
- Muise AM, Walters TD, Glowacka WK, et al. Polymorphisms in E-cadherin (CDH1) result in a mis-localised cytoplasmic protein that is associated with Crohn’s disease. Gut 2009;58:1121-7. [Crossref] [PubMed]
- Bao J, Yura RE, Matters GL, et al. Meprin A impairs epithelial barrier function, enhances monocyte migration, and cleaves the tight junction protein occludin. Am J Physiol Renal Physiol 2013;305:F714-26. [Crossref] [PubMed]
- Travassos LH, Carneiro LA, Ramjeet M, et al. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat Immunol 2010;11:55-62. [Crossref] [PubMed]
- Hoefkens E, Nys K, John JM, et al. Genetic association and functional role of Crohn disease risk alleles involved in microbial sensing, autophagy, and endoplasmic reticulum (ER) stress. Autophagy 2013;9:2046-55. [Crossref] [PubMed]
- Adolph TE, Tomczak MF, Niederreiter L, et al. Paneth cells as a site of origin for intestinal inflammation. Nature 2013;503:272-6. [Crossref] [PubMed]
- Kaser A, Lee AH, Franke A, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 2008;134:743-56. [Crossref] [PubMed]
- Willson TA, Jurickova I, Collins M, et al. Deletion of intestinal epithelial cell STAT3 promotes T-lymphocyte STAT3 activation and chronic colitis following acute dextran sodium sulfate injury in mice. Inflamm Bowel Dis 2013;19:512-25. [Crossref] [PubMed]
- Barmeyer C, Fromm M, Schulzke JD. Active and passive involvement of claudins in the pathophysiology of intestinal inflammatory diseases. Pflugers Arch 2017;469:15-26. [Crossref] [PubMed]
- Turer E, McAlpine W, Wang KW, et al. Creatine maintains intestinal homeostasis and protects against colitis. Proc Natl Acad Sci U S A 2017;114:E1273-E1281. [Crossref] [PubMed]
- Barrett JC, Hansoul S, Nicolae DL, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet 2008;40:955-62. [Crossref] [PubMed]
- Wapenaar MC, Monsuur AJ, van Bodegraven AA, et al. Associations with tight junction genes PARD3 and MAGI2 in Dutch patients point to a common barrier defect for coeliac disease and ulcerative colitis. Gut 2008;57:463-7. [Crossref] [PubMed]
- Quigley EM. Leaky gut - concept or clinical entity? Curr Opin Gastroenterol 2016;32:74-9. [Crossref] [PubMed]
- Münch A, Söderholm JD, Ost A, et al. Increased transmucosal uptake of E. coli K12 in collagenous colitis persists after budesonide treatment. Am J Gastroenterol 2009;104:679-85. [Crossref] [PubMed]
- Wildt S, Madsen JL, Rumessen JJ. Small-bowel permeability in collagenous colitis. Scand J Gastroenterol 2006;41:1044-9. [Crossref] [PubMed]
- Ocón B, Aranda CJ, Gámez-Belmonte R, et al. The glucocorticoid budesonide has protective and deleterious effects in experimental colitis in mice. Biochem Pharmacol 2016;116:73-88. [Crossref] [PubMed]
- Roth B, Manjer J, Ohlsson B. Microscopic colitis and reproductive factors related to exposure to estrogens and progesterone. Drug Target Insights 2013;7:53-62. [Crossref] [PubMed]
- Günal O, Oktar BK, Ozçinar E, et al. Estradiol treatment ameliorates acetic acid-induced gastric and colonic injuries in rats. Inflammation 2003;27:351-9. [Crossref] [PubMed]
- Pardi DS, Kelly CP. Microscopic colitis. Gastroenterology 2011;140:1155-65. [Crossref] [PubMed]
- Tachecí I, Bradna P, Douda T, et al. Small intestinal injury in NSAID users suffering from rheumatoid arthritis or osteoarthritis. Rheumatol Int 2016;36:1557-61. [Crossref] [PubMed]
- Marlicz W, Łoniewski I, Grimes DS, et al. Nonsteroidal anti-inflammatory drugs, proton pump inhibitors, and gastrointestinal injury: contrasting interactions in the stomach and small intestine. Mayo Clin Proc 2014;89:1699-709. [Crossref] [PubMed]
- Zagorowicz E, Mroz A, Kraszewska E, et al. Chronic low-dose aspirin use does not alter colonic mucosa in asymptomatic individuals: a prospective cross-sectional study J Clin Pathol 2014;67:143-52. [STROBE 1a]. [Crossref] [PubMed]
- Otani K, Tanigawa T, Watanabe T, et al. Microbiota Plays a Key Role in Non-Steroidal Anti-Inflammatory Drug-Induced Small Intestinal Damage. Digestion 2017;95:22-8. [Crossref] [PubMed]
- Endo H, Sakai E, Taniguchi L, et al. Risk factors for small-bowel mucosal breaks in chronic low-dose aspirin users: data from a prospective multicenter capsule endoscopy registry. Gastrointest Endosc 2014;80:826-34. [Crossref] [PubMed]
- Watanabe T, Tanigawa T, Nadatani Y, et al. Risk factors for severe nonsteroidal anti-inflammatory drug-induced small intestinal damage. Dig Liver Dis 2013;45:390-5. [Crossref] [PubMed]
- Freedberg DE, Lebwohl B, Abrams JA. The impact of proton pump inhibitors on the human gastrointestinal microbiome. Clin Lab Med 2014;34:771-85. [Crossref] [PubMed]
- Jackson MA, Goodrich JK, Maxan ME, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut 2016;65:749-56. [Crossref] [PubMed]
- Imhann F, Bonder MJ, Vich Vila A, et al. Proton pump inhibitors affect the gut microbiome. Gut 2016;65:740-8. [Crossref] [PubMed]
- Freedberg DE, Toussaint NC, Chen SP, et al. Proton Pump Inhibitors Alter Specific Taxa in the Human Gastrointestinal Microbiome: A Crossover Trial. Gastroenterology 2015;149:883-5.e9. [Crossref] [PubMed]
- Tariq R, Smyrk T, Pardi DS, et al. New-Onset Microscopic Colitis in an Ulcerative Colitis Patient After Fecal Microbiota Transplantation. Am J Gastroenterol 2016;111:751-2. [Crossref] [PubMed]
- Agrawal M, Aroniadis OC, Brandt LJ, et al. The Long-term Efficacy and Safety of Fecal Microbiota Transplant for Recurrent, Severe, and Complicated Clostridium difficile Infection in 146 Elderly Individuals. J Clin Gastroenterol 2016;50:403-7. [PubMed]
- Gustafsson RJ, Ohlsson B, Benoni C, et al. Mucosa-associated bacteria in two middle-aged women diagnosed with collagenous colitis. World J Gastroenterol 2012;18:1628-34. [Crossref] [PubMed]
- Fischer H, Holst E, Karlsson F, et al. Altered microbiota in microscopic colitis. Gut 2015;64:1185-6. [Crossref] [PubMed]
- Vandeputte D, Falony G, Vieira-Silva S, et al. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 2016;65:57-62. [Crossref] [PubMed]
- Falony G, Joossens M, Vieira-Silva S, et al. Population-level analysis of gut microbiome variation. Science 2016;352:560-4. [Crossref] [PubMed]
- Sapp H, Ithamukkaa S, Brien TP, et al. The terminal ileum is affected in patients with lymphocytic or collagenous colitis. Am J Surg Pathol 2002;26:1484-92. [Crossref] [PubMed]
- Caminero A, Galipeau HJ, McCarville JL, et al. Duodenal bacteria from patients with celiac disease and healthy subjects distinctly affect gluten breakdown and immunogenicity. Gastroenterology 2016;151:670-83. [Crossref] [PubMed]
- Turcotte JF, Kao D, Mah SJ, et al. Breaks in the wall: increased gaps in the intestinal epithelium of irritable bowel syndrome patients identified by confocal laser endomicroscopy (with videos). Gastrointest Endosc 2013;77:624-30. [Crossref] [PubMed]
- Hosoe N, Ogata H. Application and Efficacy of Super-Magnifying Endoscopy for the Lower Intestinal Tract. Clin Endosc 2016;49:37-40. [Crossref] [PubMed]
- Hosoe N, Ogata H, Hibi T. Endoscopic imaging of parasites in the human digestive tract. Parasitol Int 2014;63:216-20. [Crossref] [PubMed]
- Roth B, Manjer J, Ohlsson B. Microscopic Colitis is Associated with Several Concomitant Diseases. Drug Target Insights 2013;7:19-25. [Crossref] [PubMed]
- Fernández-Bañares F, de Sousa MR, Salas A, et al. Epidemiological risk factors in microscopic colitis: a prospective case-control study. Inflamm Bowel Dis 2013;19:411-7. [PubMed]
- Kao KT, Pedraza BA, McClune AC, et al. Microscopic colitis: a large retrospective analysis from a health maintenance organization experience. World J Gastroenterol 2009;15:3122-7. [Crossref] [PubMed]
- Mottacki N, Simrén M, Bajor A. Review article: bile acid diarrhoea - pathogenesis, diagnosis and management. Aliment Pharmacol Ther 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Ung KA, Gillberg R, Kilander A, et al. Role of bile acids and bile acid binding agents in patients with collagenous colitis. Gut 2000;46:170-5. [Crossref] [PubMed]
- Tysk C, Wickbom A, Nyhlin N, et al. Recent advances in diagnosis and treatment of microscopic colitis. Ann Gastroenterol 2011;24:253-62. [PubMed]
- Spijkerman M, Tan IL, Kolkman JJ, et al. A large variety of clinical features and concomitant disorders in celiac disease - A cohort study in the Netherlands. Dig Liver Dis 2016;48:499-505. [Crossref] [PubMed]
- Mellander MR, Ekbom A, Hultcrantz R, et al. Microscopic colitis: a descriptive clinical cohort study of 795 patients with collagenous and lymphocytic colitis. Scand J Gastroenterol 2016;51:556-62. [Crossref] [PubMed]
- Fritscher-Ravens A, Schuppan D, Ellrichmann M, et al. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology 2014;147:1012-20.e4. [Crossref] [PubMed]
- Kiesslich R, Duckworth CA, Moussata D, et al. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut 2012;61:1146-53. [Crossref] [PubMed]
- Winer DA, Winer S, Dranse HJ, et al. Immunologic impact of the intestine in metabolic disease. J Clin Invest 2017;127:33-42. [Crossref] [PubMed]
- Fasano A. Leaky gut and autoimmune diseases. Clin Rev Allergy Immunol 2012;42:71-8. [Crossref] [PubMed]
- Scarpignato C, Dolak W, Lanas A, et al. Rifaximin Reduces Number and Severity of Intestinal Lesions Associated With Use of Nonsteroidal Anti-Inflammatory Drugs in Humans. Gastroenterology 2017;152:980-2.e3. [Crossref] [PubMed]
- Hill C, Guarner F, Reid G, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 2014;11:506-14. [Crossref] [PubMed]
- Ducrotté P, Sawant P, Jayanthi V. Clinical trial: Lactobacillus plantarum 299v [DSM 9843] improves symptoms of irritable bowel syndrome. World J Gastroenterol 2012;18:4012-8. [Crossref] [PubMed]
- Jonkers D, Penders J, Masclee A, et al. Probiotics in the management of inflammatory bowel disease: a systematic review of intervention studies in adult patients. Drugs 2012;72:803-23. [Crossref] [PubMed]
- Zhang Y, Li L, Guo C, et al. Effects of probiotic type, dose and treatment duration on irritable bowel syndrome diagnosed by Rome III criteria: a meta-analysis. BMC Gastroenterol 2016;16:62. [Crossref] [PubMed]
- Kristensen NB, Bryrup T, Allin KH, et al. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med 2016;8:52. [Crossref] [PubMed]
- Sanders ME. Probiotics and microbiota composition. BMC Med 2016;14:82. [Crossref] [PubMed]
- van Baarlen P, Wells JM, Kleerebezem M. Regulation of intestinal homeostasis and immunity with probiotic lactobacilli. Trends Immunol 2013;34:208-15. [Crossref] [PubMed]
- Ohland CL, Macnaughton WK. Probiotic bacteria and intestinal epithelial barrier function. Am J Physiol Gastrointest Liver Physiol 2010;298:G807-19. [Crossref] [PubMed]
- Persborn M, Gerritsen J, Wallon C, et al. The effects of probiotics on barrier function and mucosal pouch microbiota during maintenance treatment for severe pouchitis in patients with ulcerative colitis. Aliment Pharmacol Ther 2013;38:772-83. [Crossref] [PubMed]
- Tromm A, Niewerth U, Khoury M, et al. The probiotic E. coli strain Nissle 1917 for the treatment of collagenous colitis: first results of an open-label trial. Z Gastroenterol 2004;42:365-9. [Crossref] [PubMed]
- Wildt S, Munck LK, Vinter-Jensen L, et al. Probiotic treatment of collagenous colitis: a randomized, double-blind, placebo-controlled trial with Lactobacillus acidophilus and Bifidobacterium animalis subsp. Lactis. Inflamm Bowel Dis 2006;12:395-401. [Crossref] [PubMed]
- Rohatgi S, Ahuja V, Makharia GK, et al. VSL#3 induces and maintains short-term clinical response in patients with active microscopic colitis: a two-phase randomised clinical trial. BMJ Open Gastroenterol 2015;2:e000018. [Crossref] [PubMed]
- Lomax AR, Calder PC. Probiotics, immune function, infection and inflammation: a review of the evidence from studies conducted in humans. Curr Pharm Des 2009;15:1428-518. [Crossref] [PubMed]
- van Hemert S, Meijerink M, Molenaar D, et al. Identification of Lactobacillus plantarum genes modulating the cytokine response of human peripheral blood mononuclear cells. BMC Microbiol 2010;10:293. [Crossref] [PubMed]