Management of strictures after endoscopic submucosal dissection for superficial esophageal cancer
Introduction
The burden between endoscopic treatment [endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD)] and surgical therapy for esophageal cancer are greatly different (1), therefore it is extremely important to find an early lesion that is an indication for endoscopic resection. Advances in image enhancement endoscopy and magnified endoscopy have increased the number of cases of early esophageal cancer detection (2), and the correct diagnosis rate for pretreatment diagnosis has also increased (3). The ESD procedure, an application of conventional EMR, has been developed and established in Japan (4). ESD has relatively high complication rates; the notification of perforation risk is essential especially in the esophagus (1,5). Bleeding during ESD can be managed by endoscopic hemostasis with soft coagulation by forceps. Even with these ESD-related incident risks taken into consideration, the merit that large lesions (i.e., superficial spreading carcinoma) can be resected en-bloc is more beneficial (6). And the number of lesions for endoscopic therapy including diagnostic treatment is increasing because of the invasiveness of surgery and chemo-radio therapy (CRT) (1). Furthermore, as the result of JCOG0508 (phase III) coming out in 2016, the relative adaptation of endoscopic therapy is expected to expand (described later).
Indication for EMR/ESD in superficial esophageal cancer
For early esophageal cancer involving the epithelium (EP) or the lamina propria (LPM), endoscopic treatment (EMR/ESD) is indicated, because of the almost nominal possibility of lymph node metastasis at that depth reaching these layers (7). For esophageal cancer invading the muscularis mucosa (MM), the lymph node metastasis rate is reported as 9%, and for cancer with shallow (<200 µm) submucosal (s-SM) invasion it increases to 19% (8). However, in these settings, there are reports that the frequency of metastasis is no less than 5% except for lesions of 50 mm or more, macroscopic type 0-I/0-III, or positive cases of vascular invasion (8), and it is regarded as a relative indication of there being lesions from EMR/ESD there (9). Table 1 shows the risk factor of lymph node metastasis in MM/s-SM esophageal cancer. In submucosal cancer that invades deeper than SM 200 µm (d-SM), metastasis is seen in 30–50% (7,8), so there is originally no indication for endoscopic therapy. But the diagnostic accuracy of MM/s-SM cancer is not satisfactory enough (3), especially for so-called superficial spreading carcinoma, it is necessary to consider a diagnostic treatment for esophageal cancer with a high surgical-related mortality rate (2–3%) (1). Furthermore, as JCOG0508 (phase III) shows, non-surgical treatments combining endoscopic resection and CRT for esophageal cancer with a suspected SM invasion were examined and proved to be effective and safe (the results shown online in Japanese). In this situation, as above, the larger lesions were also endoscopically resected by the ESD technique. Accordingly, esophageal luminal stenosis after endoscopic treatment appeared as a problem and remains unresolved.
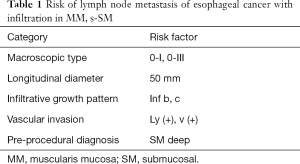
Full table
Risk and prevention of post-procedural stenosis
The risk of stenosis after esophageal ESD is primarily affected by the circumference of the resected area, and if the mucosal defect after resection exceeds 3/4 of the circumference, there is a possibility of a stricture that is clinically problematic (10). If it is resected in a complete circular (or semi-circumferential) manner, a post-procedural structure must occur. For this reason, in the 2007 edition of esophageal cancer diagnosis and treatment guidelines in Japan, an absolute indication for endoscopic therapy was described as having a circumference of less than 2/3. If larger than 2/3 the lesion is resected, and it may be necessary to dilate frequently for prevention of post-procedural stenosis. For prevention of esophageal strictures after ESD, endoscopic balloon dilation (EBD), starting from an early postoperative day, was effective, but the burden on the patients compromises their quality of life.
However, in recent years, it has been reported that the occurrence of stenosis after ESD and the frequency of required EBD sessions can be substantially decreased by local injection or oral administration of steroids (11-13), and such prophylactic ways have been widely spread in clinical practice. As a result, the limitation on the circumference of a lesion was deleted from the guidelines of esophageal cancer in the current version (9), and added on as the following comment: “When the mucosal defect is over 3/4 of circumference, the occurrence of scar stricture after mucosal resection is predicted. Therefore, sufficient preoperative explanation and prevention of stenosis are necessary.” And, it is now becoming possible to treat the whole circumference lesion via ESD and to control the stenosis (14).
Table 2 shows prevention methods of post-procedural strictures by steroids. Such treatment with steroids can be more effective than preventive EBD leading to less of a burden on patients. Currently, it is under examination by JCOG 1217 as a phase III study as to whether the steroid administration method is superior to local injections or oral administration (15). In turn, we propose that local steroid injections of triamcinolone on the day of ESD followed by oral intake of prednisolone a few days later would be “sequential steroid therapy” for post-procedural strictures against extensive excision of large esophageal cancer lesions, and is an emerging concept in single institutional preliminary trials which requires confirmation with larger prospective studies.

Full table
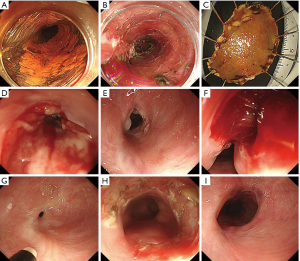
According to the method reported by Yamaguchi et al. (11), prednisolone 0.5 mg/kg/day (30 mg/day) starts to gradually taper. Oral steroid intake is easier than the local injection method and it was reported that the prevention effect of stenosis was rather high. On the other hand, there are reports that it is difficult to use in cases of diabetes, osteoporosis and certain psychiatric disorders etc. and it can develop infectious diseases that can become lethal as a result of systemic administration (16). Characteristics of steroid refractory cases are shown in Table 3 (17). Figure 1 shows our case.

Full table
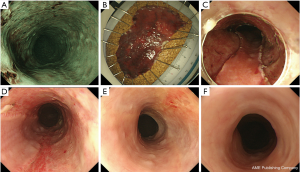
Hashimoto et al. for the first time reported a method of using a local injection of triamcinolone on days 3, 7, and 10 after ESD (12). However, it was technically difficult to locally inject steroids into the ulcer base after ESD, and there were risks such as perforation. Therefore, Hanaoka et al. reported about their results of locally pouring triamcinolone into the ulcer base for only one session immediately after ESD procedure (13), and this method is now mainstream. Figure 2 shows our case.
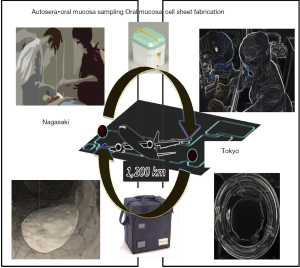
Recently, oral mucosal epithelial cell sheet transplantation applying regenerative medicine technology (18,19), and the usefulness of biodegradable stents (20) have been reported and new clinical applications are expected. In cell sheet transplantation, expensive medical expenses are required to prepare cell sheets, and there are problems to be solved such as it taking no less than 2 weeks at Tokyo Women’s Medical University Hospital. However, a clinical study of cell sheet transplantation accompanied by the transfer of a patient at Nagasaki University Hospital was conducted, and it was found to be clinically applicable to patients in remote areas and proved effective for the prevention of stenosis. The summarized results were briefly as follows. Ten patients who underwent complete circular or semicircular ESD for ESCC were transplanted with autologous oral epithelial cell sheets. The safety in every process throughout the cell sheet preparation, transport, and transplantation was confirmed. Using cell sheet transplantation, the luminal stenosis rate was 40%, while the median EBD session was 0. Median post-ESD ulcer healing period was rather short at 36 days (21). In fact, Ohki et al. have already applied endoscopic transplantation of autologous oral mucosal epithelial cell sheets in 9 patients with superficial esophageal squamous carcinoma to prevent post-ESD stricture in Advanced Biomedical Engineering and Science, Tokyo Women’s Medical University, Tokyo. Eight of the 9 patients had no experience of esophageal strictures and the procedure was safely performed without complications (19). Nevertheless, oral mucosal epithelial cell sheets transplantation has potential disadvantages. The fabrication of cell sheets is still technically and financially difficult in clinical practice even in most tertiary university hospital settings. To resolve this issue we would have to create ready-made oral mucosal epithelial cell sheets that can be transported from production facilities equipped with a cell culture facility (CCF) to a remote hospital that does not have CCFs to fabricate cell sheets, where they will be transplanted (schematic Figure 3). Then, this treatment can be performed in almost all hospitals everywhere in Japan without the necessity for the hospitals themselves to fabricate the cell sheets in their own CCF, promising regenerative medicine technology that offers a safe treatment option to prevent esophageal strictures after extensive ESD through faster healing until epithelialization. It has not yet been conclusively determined whether cell sheet transplantation is more effective for stenosis than steroid administration, and further prospective studies are required.
Treatment of post-procedural stenosis
Even for cases in which stenosis had occurred, the main treatment was frequent EBD. However, there were cases where adequate effects were not obtained, and complications such as bleeding and perforation were reported, which above all else might cause a tremendous burden on patients.
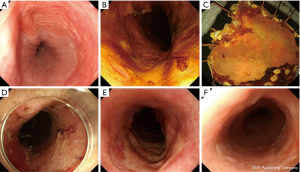
In recent years, Muto et al. reported on the usefulness of endoscopic radial incision and cutting method (ERIC) (22). This method was carried out as follows: (I) the stricture area was incised radially by using an IT knife endoscopically; (II) the virtual line that connects the esophageal lumen on the oral side and the lumen on the anal side was assumed, and an incision was performed along this line; (III) the incision area was sliced off with an IT knife; and (IV) after RIC, preventive EBD was performed repeatedly at the frequency of once per week, to maintain patency until the cutting surface became a scar. It can be expected there will be refractory stenosis after ESD, except for extremely hard scarring stenosis and long stenosis (23). Figure 4 shows our case.
Conclusions
ESD against superficial esophageal cancer has been technically capable of resecting a large lesion including wholly circumference lesions, but it remains a major task for postoperative stenosis. Although problems remain in the risk of infection and the certainty of the effect, the steroid administration method is established and the problem is being overcome by spreading it. Facilities that can administer oral mucosal epithelial cell sheet transplantation at present are limited, but clinical trials among multi-centers for using transferring cell sheets are also planned. The usefulness of the ERIC method has also been reported for refractory stenosis cases. In the future, solving the problem of stenosis after esophagus ESD will lead to the expansion of esophageal ESD indication.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Tachimori Y, Ozawa S, Numasaki H, et al. Comprehensive Registry of Esophageal Cancer in Japan, 2009. Esophagus 2016;13:110-37. [Crossref] [PubMed]
- Muto M, Minashi K, Yano T, et al. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol 2010;28:1566-72. [Crossref] [PubMed]
- Oyama T, Inoue H, Arima M, et al. Prediction of the invasion depth of superficial squamous cell carcinoma based on microvessel morphology: magnifying endoscopic classification of the Japan Esophageal Society. Esophagus 2017;14:105-12. [Crossref] [PubMed]
- Gotoda T, Kondo H, Ono H, et al. A new endoscopic mucosal resection procedure using an insulation-tipped electrosurgical knife for rectal flat lesions: report of two cases. Gastrointest Endosc 1999;50:560-3. [Crossref] [PubMed]
- Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 2009;70:860-6. [Crossref] [PubMed]
- Takahashi H, Arimura Y, Masao H, et al. Endoscopic submucosal dissection is superior to conventional endoscopic resection as a curative treatment for early squamous cell carcinoma of the esophagus (with video). Gastrointest Endosc 2010;72:255-64, 264.e1-2.
- Kodama M, Kakegawa T. Treatment of superficial cancer of the esophagus: a summary of responses to a questionnaire on superficial cancer of the esophagus in Japan. Surgery 1998;123:432-9. [Crossref] [PubMed]
- Oyama T, Miyata Y, Shimaya S. Lymph nodal metastasis of m3, sm1 esophageal cancer Stom Intest 2002;37:71-4. (in Japanese).
- Kuwano H, Nishimura Y, Oyama T, et al. Guidelines for Diagnosis and Treatment of Carcinoma of the Esophagus April 2012 edited by the Japan Esophageal Society. Esophagus 2015;12:1-30.
- Katada C, Muto M, Manabe T, et al. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc 2003;57:165-9. [Crossref] [PubMed]
- Yamaguchi N, Isomoto H, Nakayama T, et al. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc 2011;73:1115-21. [Crossref] [PubMed]
- Hashimoto S, Kobayashi M, Takeuchi M, et al. The efficacy of endoscopic triamcinolone injection for the prevention of esophageal stricture after endoscopic submucosal dissection. Gastrointest Endosc 2011;74:1389-93. [Crossref] [PubMed]
- Hanaoka N, Ishihara R, Takeuchi Y, et al. Intralesional steroid injection to prevent stricture after endoscopic submucosal dissection for esophageal cancer: a controlled prospective study. Endoscopy 2012;44:1007-11. [Crossref] [PubMed]
- Isomoto H, Yamaguchi N, Nakayama T, et al. Management of esophageal stricture after complete circular endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. BMC Gastroenterol 2011;11:46. [Crossref] [PubMed]
- Mizutani T, Tanaka M, Eba J, et al. A Phase III study of oral steroid administration versus local steroid injection therapy for the prevention of esophageal stricture after endoscopic submucosal dissection (JCOG1217, Steroid EESD P3). Jpn J Clin Oncol 2015;45:1087-90. [Crossref] [PubMed]
- Ishida T, Morita Y, Hoshi N, et al. Disseminated nocardiosis during systemic steroid therapy for the prevention of esophageal stricture after endoscopic submucosal dissection. Dig Endosc 2015;27:388-91. [Crossref] [PubMed]
- Yamaguchi N, Isomoto H, Ohnita K, et al. The prevention of stricuture arter esophageal endoscopic submucosal dissection by oral or injected steroids Endosc Dig 2012;24:91-100. (in Japanese).
- Ohki T, Yamato M, Murakami D, et al. Treatment of oesophageal ulcerations using endoscopic transplantation of tissue-engineered autologous oral mucosal epithelial cell sheets in a canine model. Gut 2006;55:1704-10. [Crossref] [PubMed]
- Ohki T, Yamato M, Ota M, et al. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology 2012;143:582-8.e1-2.
- Saito Y, Tanaka T, Andoh A, et al. Novel biodegradable stents for benign esophageal strictures following endoscopic submucosal dissection. Dig Dis Sci 2008;53:330-3. [Crossref] [PubMed]
- Yamaguchi N, Isomoto H, Fukuda H, et al. Endoscopic Strategy for Superficial Esophageal Cancer. Jpn Cancer Clin 2015;61:165-83.
- Muto M, Ezoe Y, Yano T, et al. Usefulness of endoscopic radial incision and cutting method for refractory esophagogastric anastomotic stricture (with video). Gastrointest Endosc 2012;75:965-72. [Crossref] [PubMed]
- Minamino H, Machida H, Tominaga K, et al. Endoscopic radial incision and cutting method for refractory esophageal stricture after endoscopic submucosal dissection of superficial esophageal carcinoma. Dig Endosc 2013;25:200-3. [Crossref] [PubMed]


