Helping prometheus: liver protection in acute hemorrhagic shock
Traumatic hemorrhagic shock is considered the most common cause of death among young people in industrialized countries. Injuries to large vessels cause hypovolemia and generalized ischemia. This leads to microcirculatory disturbances, metabolic changes, and depression of the immune system (1-5). In addition, it is associated with low organ perfusion and an imbalance between O2 demand and O2 supply (6). The reaction of the body is the activation of leukocytes and macrophages and an excessive production and release of various humoral and cellular mediators including cytokines and eicosanoids as well as (7,8). To compensate the progressive loss of blood, the adrenergic system is activated. The consequence is a peripheral vasoconstriction and a reduction in the flow velocity of the blood due to deterioration in the flow properties. The disturbance of the microcirculation triggers tissue hypoxia and acidosis (9-11). A redistribution of the circulating blood volume primarily to vital organs such as the brain and heart is the consequence. By this, the perfusion organs such as liver, kidney and gastrointestinal tract are further reduced, because they are not included in the hypovolemia-induced centralization of blood perfusion. The insufficient supply to these organs results in distinct microcirculation disorders, which, in the short-term, causes tissue and organ damage and which can further lead to the development of multiple organ failure (10).
Stages and consequences of hemorrhagic shock
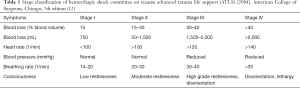
Based on the estimated blood loss and the resulting consequences of hemorrhagic shock in accordance with the guidelines of the American College of Surgeons for Advanced Trauma Life Support (ATLS) hemorrhagic shock can be divided into four stages (12) (Table 1). Stage I is classified at a loss of 15% of the circulating blood volume. Heart rate and blood pressure are in this case still normal. Initial symptoms may be thirst and restlessness. Stage II is defined as a blood loss of 15–30%. Clinical symptoms here are tachycardia, tachypnea, oliguria and moderate agitation. A correction of this state can be achieved with fluid resuscitation or vasopressor support. Stage III of hemorrhagic shock is the category with a blood loss of 30–40%. Symptoms include a pronounced tachycardia and tachypnea, hypotension, severe restlessness and disorientation. A blood loss of >40% represents stage IV of hemorrhagic shock. Characteristic for this state are, for example, lethargy, disorientation, tachycardia, and tachypnea. The gastrointestinal tract reacts particularly sensitive to shock-induced alterations of blood perfusion, mainly involving the tissue supplied by the superior mesenteric artery (13). The ischemia caused by the hemorrhagic shock can lead to damage of the intestinal mucosal barrier and subsequent invasion of bacteria and endotoxins from the intestinal lumen into the mesenteric vessels (14). The invading bacteria enter the liver through the portal circulation and cause a release of mediators (especially IL-6, IL-1, TNF-α and corticoids) and radicals by macrophages, monocytes, endothelial cells and fibroblasts. The release of these mediators has significant effects on the liver microcirculation and on hepatocyte function (7,8,15). The acute phase reaction is controlled by mediators, which affect protein synthesis of the liver (16,17). The cytokines released as a result of the hemorrhagic shock induce the synthesis of albumin, C-reactive protein, complement 5a, ceruloplasmin, transferrin, α1-acid glycoprotein and haptoglobin, responsible for controlling the coagulation and fibrinolysis (17). Hemorrhagic shock affects all the cell systems of the liver: The number of activated Kupffer cells and Ito cells is increased and the number of damaged endothelial cells rises. This results in disorders of the liver microcirculation, in particular in a disturbance of sinusoidal perfusion and a further dysregulation of hepatocyte function (18-20). Significant changes in liver-specific aspartate aminotransferase can be detected in an animal shock model (21) after 3 hours in serum. In hemorrhagic shock, there is a drop in cardiac output and a disproportionately greater decrease in hepatic blood flow due to blood loss. The consequence is failure of hepatic microcirculation and reduced perfusion pressure due to the reduced volume of circulating blood in the body. This can lead to perfusion failure of individual liver sinusoids. Simultaneous adrenergic activation also leads to a reduction of the sinusoidal diameter with a consequent deficit in perfusion (20). The result is failure of the sinusoidal network of the liver. Under normotensive conditions circulating leukocytes flow freely through the sinusoids of the liver, evoking no interactions with the vascular endothelium. Circulatory disturbance results in sinusoidal congestion with increased leukocyte activation (21,22) as well as increased leukocyte endothelial interactions (7).
Full table
Treatment
To counteract acute hemorrhagic shock, the use of intravenous infusions such as colloidal and crystalloid solutions, are in use. Decreased volume is restored, decreased blood pressure is raised quickly to normotensive values and the circulatory status of the injured patients is stabilized. An increase in the venous return to the heart with the same resistance-related increase in cardiac output shall maintain the perfusion of vital organs (23-25). Small volume resuscitation (SVR) treatment has become the choice for primary therapy. SVR is defined as a rapid intravenous infusion of small hyperosmolar saline colloid solutions, which improves microcirculation and oxygen supply in the tissue. Vasopressors can support peripheral vascular resistance in acute hemorrhagic shock. Well-known in this context are the two catecholamines adrenaline and noradrenaline which are produced in the adrenal medulla and specifically act on α and β receptors of the vessels. A release occurs especially in stressful situations. However, the main assessment criterion is adrenaline (26-28). Studies show that the use of epinephrine in cardiac arrest does not lead to an improvement in short-term survival. An increase in adrenaline dosage can even result in poorer neurological performance (28). Another vasopressor is the body hormone vasopressin (29), which has proven itself as a successful vasopressor in the case of vasodilatory shock (30-32). Thus, vasopressin improves organ blood flow during hemorrhagic shock even without any catecholamine effect and in the presence of vasoplegia.
Experimental approaches
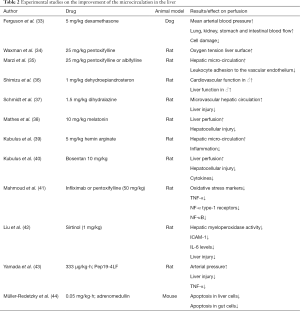
There are several experimental animal models, which enable the study of improved microcirculation in the liver after application of drugs in acute hemorrhagic shock (Table 2). These particular animal models are mainly applied to rats, hamsters, sheep, dogs, pigs and—to a lesser degree—also mice. Common techniques to study the microcirculation are laser-Doppler flux-metry (21) and intravital microscopy (45). A study in 1978, which was carried out on dogs, showed that the use of 5 mg of dexamethasone (33) per kilogram of body weight after hemorrhagic shock leads to an improvement of the mean arterial blood pressure. The result is an increase in lung, kidney, colon and stomach perfusion, with a consequent reduction in cell damage. In 1987 the group of Waxman et al. (34) showed that the use of pentoxifylline (25 mg/kg) induces a significant increase in the oxygen tension at the surface of the liver of rats. Marzi et al. (35) showed that, after hemorrhagic shock, the use of 25 mg/kg pentoxifylline or Albifylline results in a significant improvement of microvascular blood flow to the liver. A decrease in leukocyte endothelial cell interaction on the vessel wall was also detected using intravital microscopy. Also Flynn et al. found restoration of the hepatic microcirculation using pentoxifylline (46). Shimizu et al. (36) showed that the use of dehydroepiandrosterone (1 mg/kg) in male rats after hemorrhagic shock leads to reduced bile production as well as a lower production of the serum alanine aminotransferase, liver nitrite/nitrate, the nitric oxide synthase (iNOS) and of endothelin-1 in comparison with non-treated animals. Dehydroepiandrosterone therefore may be active in the restoration of liver function. Schmidt et al. were able to show that by using the vasodilator hydralazine (1.5 mg/kg) the hepatic microvascular blood flow can be increased after acute hemorrhagic shock (37). A study confirmed a subsequent reduction in liver damage in rats. Mathes et al. demonstrated reduced hepatocellular injury in rats subjected to hemorrhagic shock when pre-treatment with melatonin (10 mg/kg) was carried out (38). Moreover, an improved perfusion was observed compared to the control group. Pre-treatment of rats with hemin arginate (5 mg/kg) showed a significant improvement of the microcirculation in the liver (39). It is presumed that the activation of the hematoxygenase-1 is responsible for this; however the exact mechanism is not yet known. Kubulus et al. examined the influence of hemoglobin glutamer-200 and its effect on the release of endothelin-1 after acute hemorrhagic shock (40). A test group rats received hemoglobin glutamer-200 after hemorrhagic shock. Enhanced expression of endothelin-1 with an increase in hepatocellular damage was thereby determined. A second experimental group was additionally administered non-specific endothelin receptor blocker bosentan (10 mg/kg). This blockade resulted in improved liver perfusion, decreased hepatocellular damage and reduced release of cytokine. Mahmoud et al. showed that in rats after hemorrhagic shock, inhibition of tumor necrosis factor-alpha (TNF-α) by pentoxifylline or infliximab (50 mg/kg) leads to a significant reduction in liver damage (41). Responsible for this seems to be a decrease in oxidative stress markers, a reduction in the expression of TNF-α, TNF-α-type-1 receptors, and the nuclear factor kappa B (NF-kB). Thus, TNF-α inhibition could be a therapeutic intervention in acute hemorrhagic shock. Finally, Liu et al. showed that levels of interleukin-6, intracellular adhesion molecule-1 (ICAM-1) as well as myeloperoxidase activity which had increased after hemorrhagic shock, were reduced by the application of sirtinol (1 mg/kg) (42). This was detected in a rat model of hemorrhagic shock. However, the exact mechanism for the anti-inflammatory effect of sirtinol has not yet been clarified. Again, in rats very recently a synthetic antimicrobial peptide was found to be protective against organ injury in a severe hemorrhagic shock (43).
Full table
A superior shock model is the Staub’s sheep. The use of smaller animals such as rats, guinea pigs and dogs affords financial and man power advantages compared to large animals. Using cutting-edge techniques such as intravital microscopy, Doppler sonography and magnetic resonance imaging changes in leukocyte function, macrophage activity and the microcirculation in the liver were observed in acute hemorrhagic shock.
Recent animal studies have shown that judicious use of drugs (Table 2) improves microcirculation and reduces liver damage after acute hemorrhagic shock. Benefits of immune modulatory substances, seen in animal studies, still have not solved the clinical problem (47,48). A hot candidate for the treatment of liver failure in the context of hemorrhagic shock is adrenomedullin. Adrenomedullin is an endogenous peptide which protected against liver and gut injury which were induced by mechanical ventilation in mice with pneumonia (44). Future studies should in particular concentrate on mechanisms attenuating inflammation and retrieving organ function in order to achieve a significant reduction in the high mortality after acute hemorrhagic shock and severe blood loss.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pfeifer R, Tschernig T, Lichte P, et al. MALP-2 pre-treatment modulates systemic inflammation in hemorrhagic shock. J Inflamm (Lond) 2013;10:17. [Crossref] [PubMed]
- Chaudry IH, Wichmann MW, Ayala A. Immunological alterations following hemorrhagic shock: Considerations for resuscitation with blood substitutes." In: Rabinovici R, Feuerstein G, Randolph AS. editors. Fundamental Principles and Clinical Applications of Blood Substitutes. New York: Marcel Dekker, 1997:165-88.
- DeCamp MM, Demling RH. Posttraumatic multisystem organ failure. JAMA 1988;260:530-4. [Crossref] [PubMed]
- Shires GT. Response to "Delayed fluid resuscitation in hemorrhagic shock induces proinflammatory cytokine response". Ann Emerg Med 2007;50:354; author reply 354. [Crossref] [PubMed]
- Jiang JX, Diao YF, Tian KL, et al. Effect of hemorrhagic shock on endotoxin-inducing TNF production and intra-tissue lipopolysaccharide-binding protein mRNA expression and their relationship. Shock 1997;7:206-10. [Crossref] [PubMed]
- Adams HA, Baumann G, Gänsslen A, et al. Definition of shock types. Anasthesiol Intensivmed Notfallmed Schmerzther 2001;36 Suppl 2:S140-3. [Crossref] [PubMed]
- Marzi I, Bauer C, Hower R, et al. Leukocyte-endothelial cell interactions in the liver after hemorrhagic shock in the rat. Circ Shock 1993;40:105-14. [PubMed]
- Marzi I, Bauer M, Secchi A, et al. Effect of anti-tumor necrosis factor alpha on leukocyte adhesion in the liver after hemorrhagic shock: an intravital microscopic study in the rat. Shock 1995;3:27-33. [PubMed]
- Nuytinck HK, Offermans XJ, Kubat K, et al. Whole-body inflammation in trauma patients. An autopsy study. Arch Surg 1988;123:1519-24. [Crossref] [PubMed]
- Keel M, Trentz O. Pathophysiology of polytrauma. Injury 2005;36:691-709. [Crossref] [PubMed]
- Messmer K. Rheologic principles of shock therapy. Internist (Berl) 1982;23:445-9. [PubMed]
- American College of Surgeons. ATLS Advanced Trauma Life Support Program for Doctors (7th Ed.). Chicago: American College of Surgeons, 2014.
- Messmer K. 163. Intestinal factors in shock: intestinal circulation. Langenbecks Arch Chir 1967;319:890-909. [Crossref] [PubMed]
- Berg RD. Bacterial translocation from the gastrointestinal tract. J Med 1992;23:217-44. [PubMed]
- Swank GM, Deitch EA. Role of the gut in multiple organ failure: bacterial translocation and permeability changes. World J Surg 1996;20:411-7. [Crossref] [PubMed]
- Rose S, Marzi I. Pathophysiology of polytrauma. Zentralbl Chir 1996;121:896-913. [PubMed]
- Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J 1990;265:621-36. [Crossref] [PubMed]
- Vollmar B. Microcirculation and oxygen supply to the liver in hemorrhagic shock and sepsis. Anasthesiol Intensivmed Notfallmed Schmerzther 1995;30 Suppl 1:S52-4. [Crossref] [PubMed]
- Mazzoni MC, Borgström P, Intaglietta M, et al. Capillary narrowing in hemorrhagic shock is rectified by hyperosmotic saline-dextran reinfusion. Circ Shock 1990;31:407-18. [PubMed]
- Koo A, Liang IY. Blood flow in hepatic sinusoids in experimental hemorrhagic shock in the rat. Microvasc Res. 1977;13:315-25. [Crossref] [PubMed]
- Tang Y, Xia XF, Zhang Y, et al. Establishment of an experimental mouse model of trauma-hemorrhagic shock. Exp Anim 2012;61:417-25. [Crossref] [PubMed]
- Clemens MG, Bauer M, Pannen BH, et al. Remodeling of hepatic microvascular responsiveness after ischemia/reperfusion. Shock 1997;8:80-5. [Crossref] [PubMed]
- Kreimeier U, Christ F, Frey L, et al. Small-volume resuscitation for hypovolemic shock. Concept, experimental and clinical results. Anaesthesist 1997;46:309-28. [Crossref] [PubMed]
- Marik PE. Fluid Responsiveness and the Six Guiding Principles of Fluid Resuscitation. Crit Care Med 2016;44:1920-2. [Crossref] [PubMed]
- Marik PE, Linde-Zwirble WT, Bittner EA, et al. Fluid administration in severe sepsis and septic shock, patterns and outcomes: an analysis of a large national database. Intensive Care Med 2017;43:625-32. [Crossref] [PubMed]
- Paradis NA, Koscove EM. Epinephrine in cardiac arrest: a critical review. Ann Emerg Med 1990;19:1288-301. [Crossref] [PubMed]
- Woodhouse SP, Cox S, Boyd P, et al. High dose and standard dose adrenaline do not alter survival, compared with placebo, in cardiac arrest. Resuscitation 1995;30:243-9. [Crossref] [PubMed]
- Behringer W, Kittler H, Sterz F, et al. Cumulative epinephrine dose during cardiopulmonary resuscitation and neurologic outcome. Ann Intern Med 1998;129:450-6. [Crossref] [PubMed]
- Lindner KH, Prengel AW, Pfenninger EG, et al. Vasopressin improves vital organ blood flow during closed-chest cardiopulmonary resuscitation in pigs. Circulation 1995;91:215-21. [Crossref] [PubMed]
- Krismer AC, Wenzel V, Mayr VD, et al. Arginine vasopressin during cardiopulmonary resuscitation and vasodilatory shock: current experience and future perspectives. Curr Opin Crit Care 2001;7:157-69. [Crossref] [PubMed]
- Dünser M, Wenzel V, Mayr AJ, et al. Arginine vasopressin in vasodilatory shock: a new therapy approach? Anaesthesist 2002;51:650-9; discussion 659-60. [Crossref] [PubMed]
- Morales D, Madigan J, Cullinane S, et al. Reversal by vasopressin of intractable hypotension in the late phase of hemorrhagic shock. Circulation 1999;100:226-9. [Crossref] [PubMed]
- Ferguson JL, Roesel OF, Bottoms GD. Dexamethasone treatment during hemorrhagic shock: blood pressure, tissue perfusion, and plasma enzymes. Am J Vet Res 1978;39:817-24. [PubMed]
- Waxman K, Holness R, Tominaga G, et al. Pentoxifylline improves tissue oxygenation after hemorrhagic shock. Surgery 1987;102:358-61. [PubMed]
- Marzi I, Maier M, Herzog C, et al. Influence of pentoxifylline and albifylline on liver microcirculation and leukocyte adhesion after hemorrhagic shock in the rat. J Trauma 1996;40:90-6. [Crossref] [PubMed]
- Shimizu T, Szalay L, Choudhry MA, et al. Mechanism of salutary effects of androstenediol on hepatic function after trauma-hemorrhage: role of endothelial and inducible nitric oxide synthase. Am J Physiol Gastrointest Liver Physiol 2005;288:G244-50. [Crossref] [PubMed]
- Schmidt R, Baechle T, Hoetzel A, et al. Dihydralazine treatment limits liver injury after hemorrhagic shock in rats. Crit Care Med 2006;34:815-22. [Crossref] [PubMed]
- Mathes AM, Kubulus D, Pradarutti S, et al. Melatonin pretreatment improves liver function and hepatic perfusion after hemorrhagic shock. Shock 2008;29:112-8. [PubMed]
- Kubulus D, Mathes A, Pradarutti S, et al. Hemin arginate-induced heme oxygenase 1 expression improves liver microcirculation and mediates an anti-inflammatory cytokine response after hemorrhagic shock. Shock 2008;29:583-90. [PubMed]
- Kubulus D, Mathes A, Reus E, et al. Endothelin-1 contributes to hemoglobin glutamer-200-mediated hepatocellular dysfunction after hemorrhagic shock. Shock 2009;32:179-89. [Crossref] [PubMed]
- Mahmoud MF, El Shazly SM, Barakat W. Inhibition of TNF-α protects against hepatic ischemia-reperfusion injury in rats via NF-κB dependent pathway. Naunyn Schmiedebergs Arch Pharmacol 2012;385:465-71. [Crossref] [PubMed]
- Liu FC, Tsai YF, Yu HP. Sirtinol attenuates trauma hemorrhage-induced hepatic injury through Akt-dependent pathway in rats. J Trauma Acute Care Surg 2013;74:1027-32. [Crossref] [PubMed]
- Yamada N, Martin LB, Zechendorf E, et al. Novel Synthetic, Host-defense Peptide Protects Against Organ Injury/Dysfunction in a Rat Model of Severe Hemorrhagic Shock. Ann Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Müller-Redetzky HC, Will D, Hellwig K, et al. Mechanical ventilation drives pneumococcal pneumonia into lung injury and sepsis in mice: protection by adrenomedullin. Crit Care 2014;18:R73. [Crossref] [PubMed]
- Menger MD, Marzi I, Messmer K. In vivo fluorescence microscopy for quantitative analysis of the hepatic microcirculation in hamsters and rats. Eur Surg Res 1991;23:158-69. [Crossref] [PubMed]
- Flynn WJ, Cryer HG, Garrison RN. Pentoxifylline but not saralasin restores hepatic blood flow after resuscitation from hemorrhagic shock. J Surg Res. 1991;50:616-21. [Crossref] [PubMed]
- Deitch EA. Animal models of sepsis and shock: a review and lessons learned. Shock 1998;9:1-11. [Crossref] [PubMed]
- Ertel W, Morrison MH, Ayala A, et al. Anti-TNF monoclonal antibodies prevent haemorrhage-induced suppression of Kupffer cell antigen presentation and MHC class II antigen expression. Immunology 1991;74:290-7. [PubMed]


