Morbidity and mortality associated with obesity
Clinical context
Obesity and overweight are defined as a systemic disease that shows excessive and abnormal accumulation of body fat leading to adverse health effects. Obesity imposes devastating health and financial tolls on individuals and society. Despite significant efforts to increase awareness, the obesity epidemic continues at an alarming rate (1).
More than half of the European population is overweight and up to 30% is obese with prevalence worldwide doubling since 1980 [World Health Organization (WHO) 2011] (2). Obesity is associated with higher rates of death driven by comorbidities such as type 2 diabetes mellitus (T2DM), dyslipidemia, hypertension, obstructive sleep apnea (OSA), certain types of cancer, steatohepatitis, gastroesophageal reflux, arthritis, polycystic ovary syndrome (PCOS), and infertility (3).
Categorising of body weight
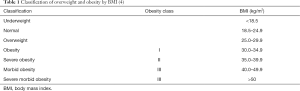
There are three measures of obesity often used in epidemiological studies: body mass index (BMI), waist circumference (WC) and waist to hip circumference ratio (WHR). The most commonly used is BMI which equals the ratio of weight in kilograms divided by height in meters squared (kg/m2). The classes of BMI reported by the WHO are, 18.5–24.9 kg/m2 for normal, 25.0–29.9 kg/m2 for overweight and >30 kg/m2 for obesity (Table 1) (4).
WC and WHR can be used to measure abdominal obesity (5) and its association with various metabolic risk factors which appear more useful than the use of BMI alone (6,7). The cut-off of WC (>102 cm in men and >88 cm in women) is difficult to apply in all populations as people from Asia appear to have higher morbidity at lower cut-off for WC compared to White Caucasians (8).
Genes and environmental—the aetiology of obesity
Obesity is underpinned by positive energy balance believed to be driven by hyperphagia arising as a consequence of increased hunger, decreased satiety or both. Pathology of the subcortical areas of the brain that control appetite is influenced by environmental factors superimposed on genetically determined susceptibility. Although ‘fatness’ runs in families, it has been difficult to separate the influences of nature versus nurture. Heritable factors account for approximately 70% of the difference in BMI in adult life (9,10). Body composition, distribution of fat and visceral fat deposition after periods of overeating share a similar genetic component (11). Environmental factors include marketing, advertising, increasing portion sizes, accessibility and availability of calorie dense foods and increased automation, all of which have contributed to increased energy intake and reduced energy expenditure (12).
Obesity and its major co-morbidities
Diabetes
The term of “diabesity” is used to describe the overlap between T2DM and obesity (13). About 50% of diagnosed diabetic patients are obese, but only approximately 20% of patients seeking bariatric surgery are diabetic (14,15). The risk of developing T2DM increases by 20% for each 1 kg/m2 increased in the BMI (16). The risk of T2DM does not increase up to a BMI <27.2 kg/m2. But a BMI of 27.2 to 29.4 kg/m2 the risk will rise by 100% and increases by about 300% for BMI >29.4 kg/m2 (5,16).
Pathogenesis
Obesity is associated with elevated circulating free fatty acids (FFAs) (17), which induce oxidative stress by promoting the production of reactive oxygen species (ROS) to a level greater than their removal, and the high level of ROS is the main cause of insulin resistance (18,19). A high-fat diet is associated with a reduction in the hepatic levels of the antioxidant glutathione (GSH) and diminished activity of antioxidant enzymes, while the activity of some enzymes such as NADPH oxidase which produce ROS, is augmented (20,21). In skeletal muscle, markers of oxidative stress have also been reported to be increased by high-fat diet which leads to increased peripheral insulin resistance in association with ectopic fat storage in muscle (21,22). With time the pancreas becomes exhausted and blood glucose level begins to increase as not enough insulin is produced to overcome the resistance. Once hyperglycemia occurs, its toxic effect on islet cells (glucotoxicity) exacerbates the problem (23). Consequently, the increase in FFAs causes lipotoxicity. Insulin resistance at the muscular, hepatic and adipose tissues increases proinflammatory cytokines and decreases anti-inflammatory cytokines, resulting in chronic inflammation.
The long-term complications of T2DM include cardiovascular diseases (CVD), stroke, peripheral vascular diseases (PVD), retinopathy, nephropathy, neuropathy (23). Therefore, prevention or at least control of T2DM will reduce complications and direct healthcare costs of obesity.
CVD
Obesity associated with the metabolic syndrome is associated with CVD (24). Metabolic syndrome is defined as a combination of at least three of the following features: central obesity, high serum triglyceride (TG) levels, low serum high-density lipoprotein (HDL), cholesterol levels, hypertension, and elevated fasting blood glucose levels. Cardiomyopathy associated with obesity is characterized by left ventricular hypertrophy and diastolic dysfunction (25). The prolonged exposure to obesity leads to worsening of cardiac function and larger ventricular mass (26), while left atrial dilatation and systolic dysfunction may also develop (27). Obesity also increases the risk of atrial fibrillation, but not stroke (28). Obese patients are 3.5 times more likely to have hypertension, while 60–70% of hypertension in adults may be attributable to adiposity (29). In a meta-analysis study of 2.88 million individuals, obesity was associated with an increase in mortality rate, with a hazard ratio of 1.18 (95% CI, 1.12–1.25) (30).
Airway
Severe obesity can be associated obesity-hypoventilation Syndrome (OHS) which defined as; the combination of obesity and chronic daytime hypercapnia [arterial carbon dioxide pressure (PaCO2) ≥45 mmHg] (31,32). The prevalence of OHS is 0.3–0.4% of the general population in Western countries (33,34) and 10–20% in patients with obesity associated obstructive sleep apnoea (35,36), to almost 50% of hospitalized patients with a BMI greater than 50 kg/m2 (37). OHS associated with higher morbidity and mortality than either OSA or simple obesity (38). Using data from seven hospitals in Akashiba et al. study showed that hypercapnia was 9% in OSA non-obese patients and 32% in obese OSA patients (39). The survival rate for continuous positive airway pressure (CPAP)-treated obstructive sleep apnoea syndrome (OSAS) patients in the Campos-Rodriguez et al. study was 85.5% (40). However, it was 77.3% for the treated OHS patients in Priou et al. cohort study (41). So, we can conclude that mortality is higher in OHS than in OSAS as demonstrated by Castro-Añón et al. who showed that patients with OHS had a 2-fold increase in the risk of mortality compared with those with OSAS (42).
BMI showed a strong, independent and positive relation with asthma possibly because of the impact of adipose tissue on the chest wall causing a restrictive lung disease, but also as obesity is associated with a chronic inflammatory state (43,44). The pulmonary function tests (PFTs) of obese patients often show impaired expiratory reserve volume (ERV), functional residual capacity (FRC) and total lung capacity (TLC), secondary to increased abdominal load and disturbed chest wall mechanics (45,46).
Kidney
Obesity is associated with abnormal renal parameters, obesity-related glomerulopathy, and chronic kidney disease (CKD). Obese patients often have increased albumin excretion rates (AER) that indicate early renal impairment and elevated risk of cardiovascular (CV) morbidity and mortality (47-49). Microalbuminuria prevalence correlates positively with total and central adiposity even in the absence of diabetes and hypertension (50,51). For each 5 kg/m2 increase in BMI, mortality associated with kidney diseases increases by 60% (52). Furthermore, the dyslipidemia which associated with obesity leading to progressive CKD by promoting inflammation and endothelial dysfunction (53). Lower concentrations of HDL, are associated with a higher incidence of CKD in the general population (54). Evidence for a link between dyslipidemia and reduced renal function in children was demonstrated in a population-based study from Turkey (CREDIT-C study), where both hypercholesterolaemia and a higher BMI were associated with a lower glomerular filtration rate (47).
Liver
Nonalcoholic fatty liver disease (NAFLD) includes hepatic steatosis, non-alcoholic steatohepatitis (NASH), fibrosis, and cirrhosis. NAFLD is the most common cause of chronic liver disease in the United States (55,56). Obesity and insulin resistance are considered to be the main causative factors of NAFLD; the percentage of obese patients seeking bariatric surgery that have steatosis is 91%; 37% have NASH and 10% show cirrhosis (57). Hepatic steatosis arises from an imbalance between TG production and utilization. The FFAs used for the hepatic production of TG are derived from diet, de novo lipogenesis (DNL) and adipose tissue lipolysis. Approximately 60% of accumulated TG in the livers of NAFLD patients are derived from FFAs mobilized from peripheral adipose depots, 25% from DNL, and the remaining 15% from dietary lipids (58).
Based on the Argo and colleagues analysis of ten studies, 37.6% of NAFL patients have progressive fibrosis over 5.3 years (59). The incidences of hepatocellular carcinoma were 4%, 10%, 3.6%, and 8.9% in Ascha et al., Ratziu et al., Bugianesi et al., and Hashimoto et al. studies respectively (60-63).
Gonadal
Obesity is associated with subfertility (64) with up to 41.9% of women seeking obesity surgery suffering from subfertility, the subfertility in obese patients in this cohort study attributed to androgen excess, insulin resistance, and hyperinsulinism (65). Obesity has marked effects on sex hormone secretion and metabolism changing the bioavailability of estrogen and androgens.
With increasing adiposity, there is an increase in peripheral aromatization of androgens to estrogens associated with a decrease in the hepatic synthesis of sex hormone-binding globulin (SHBG), which cause an increase in free oestradiol and testosterone. This is further exacerbated by an associated hyperinsulinemia resulting in decreased SHBG and stimulation of ovarian androgen production. Excess secretion of luteinizing hormone (LH) and the increased androgen to estrogen ratio and the overall altered endocrine milieu, in turn, lead to abnormal folliculogenesis and follicular atresia (66). Analysis of follicular fluid assayed for various hormones and metabolites from patients undergoing in vitro fertilization (IVF) cycles demonstrate marked differences between obese and non-obese patients. High level of mediators such as C-reactive protein, interleukin-6, tumor necrosis factor-α and plasminogen activator inhibitor type-1 in obese patients have a deleterious effect on the reproductive cycle (67).
Gastroesophageal reflux
The prevalence of gastroesophageal reflux disease (GERD) is estimated to be 20–44% in the Western countries, with a lower frequency in Asia (68). In particular, this escalation is suggested to be related to the global rise in obesity (69,70). The cause for this increasing prevalence is uncertain. Dietary changes with increasing fat intake, smoking, alcohol use, and possibly decrease in the prevalence of Helicobacter pylori infection are possibilities (71). Hampel et al. study which analyzed nine different studies reported that there are statistically significant associations between BMI and GERD in six studies, significant associations of BMI with erosive esophagitis in six studies, and significant associations of BMI with gastric cardia adenocarcinoma in four studies (72).
BMI >30 kg/m2 (as compared with BMI <25) is associated with increased acid reflux episodes, long reflux episodes (>5 min), pH <4 in the oesophagus, and a calculated summary score. The association between reflux parameters and BMI was largely mediated by WC (71).
Cancer
Obesity increases cancer incidence (73) partly by converting high-fat diet supplied fatty acids or de novo synthesized fatty acids into protumorigenic signaling lipids. Signaling lipids then signal onto the cancer cell through paracrine or autocrine interactions, while aggressive cancer cells upregulate monoacylglycerol lipase to generate fatty acids. These are incorporated in oncogenic signaling lipids that in-turn drives cancer pathogenicity (74).
Obesity is associated with an increased risk of developing insulin resistance which considered a major metabolic abnormality in most patients with type 2 diabetes characterized by elevated levels of circulating insulin (75). A recent meta-analysis of observational studies has revealed that insulin resistance and hyperinsulinemia is a significant risk factor for endometrial cancer (76).
Premenopausal women mainly synthesize estrogens in the ovary. However, in postmenopausal women ovarian biosynthesis is replaced by peripheral site synthesis, and in obese postmenopausal women, adipose tissue is the main source of estrogen biosynthesis. So, obese postmenopausal women have significant increases in estrone, estradiol, and free estradiol (77,78). This mechanism of estrogen production can lead to local estrogen levels in breast tumors that are as much as 10-fold higher compared with the circulation. In fact, a recent publication assessing breast cancer risk factors listed BMI and weight gain between the ages of 20 and 50 years as second only to Gail Model parameters [quantitative breast density, free estradiol, parity (yes/no), and age of menopause] in importance (79). The association between obesity and breast cancer risk is complex, varying by breast cancer subtype and by menopausal status. Although obesity is associated with reduced breast cancer incidence in premenopausal women (80), it is associated with increased breast cancer incidence in postmenopausal women (80,81). Obesity acts as a protective factor for premenopausal breast cancer, but it is associated with increased risk of breast cancer subtypes such as triple negative and basal-like disease (82). Obesity is associated with increased risk of breast cancer recurrence and mortality in both pre and postmenopausal women (83,84). Multiple studies have demonstrated that this association may be stronger in women with hormone receptor-positive tumors (85-87).
Medication
The comorbidities of obesity necessitate an increase in drug prescriptions for CVD, pain, psychiatric disorders, diabetes mellitus, and asthma (88). Narbro et al.’s study demonstrates that 52% of the obese individuals were taking medications compared with only 36% of the randomly selected reference populations (89). Physiological changes such as increased muscle mass, connective tissue, and total body water in obese patients alter pharmacokinetics and pharmacodynamics of medications (90).
Functional (physical functioning)
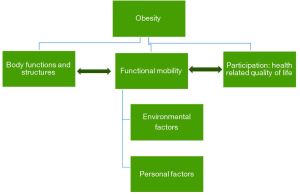
The severely obese patient shows marked impairment in their activities of daily living such as walking, climbing stairs, and bathing. These problems are often very distressing (91,92), which leading to increasing the risk of musculoskeletal pain and osteoarthritis (93). Figure 1 illustrates how mobility disability and functioning are viewed as an outcome of the interactions between obesity, body functions, structures, personal and environmental factors (94). Obesity is associated with decreased postural control and stability which hinders the individual’s ability to adapt to changes in terrain or grades during walking. One of the etiological factors for this is the abnormal distribution of body fat in the abdominal area. This leads to a forward anterior-posterior (AP) center of pressure; meaning that they carry their weight toward the front of their feet, and AP instability during static and dynamic balance (95). The instability created by abnormal body fat distribution mainly compensated by the changes to temporospatial gait parameters (distance between steps and number of steps per minute) (96).
Perceived health status
Around 25–30% of obese patients seeking bariatric surgery show marked clinical symptoms of depression. According to the WHO, depression is considered one of the main causes of disability, affecting about 121 million people all over the world (97).
Body image
Defined as the mental image a person has about the size, shape, and form of his/her body, as well as the feelings about these characteristics and constituent body parts. Body dissatisfaction (BD) is the discrepancy between the real and an individual’s idealized body image (98,99). Decreased physical activities in obese patients make them depressed, socially isolated, or discriminated against; resulting in poor self-esteem, body image distortions, and making them more likely to be the targets of teasing or bullying (100,101).
Economic repercussions
Reduced productivity, unemployment, and direct healthcare costs are the main economic repercussions of obesity (102). In the UK, healthcare costs associated with obesity account for 2.3–2.6% of all public health spending (103), Studies of the economic impact of obesity sometimes examine direct costs, while others focus on indirect costs or both. Direct costs refer to money consumed to treat obesity-related health problems such as hospitalization, medical consultations in outpatient clinics and the consumption of medications, while indirect costs refer to lost productivity or costs to the economy outside of the health sector.
Mortality associated with obesity
Obesity is associated with an increased risk of disease and death, particularly from CVD and cancer (52,104). The Association between BMI and mortality substantially varies between populations and causes of death (52,105) and can change over time (106,107).
Regarding the study of Flegal et al.: relative to normal weight, grade 2 and 3 obesity were both associated with significantly higher all-cause mortality. Grade 1 obesity was not associated with higher mortality, suggesting that the excess mortality in obesity may predominantly be due to elevated mortality at higher BMI levels (108).
Kings Obesity Staging Criteria (KOSC)
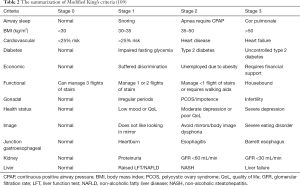
The KOSC (109) was designed to allow a practical and simplified assessment of obesity-related complications and risk of mortality to minimize inter-operator variability. It grades 12 aspects of obesity-related morbidity in line with those described above, and indexed in alphabetical order to improve ease of use: airways, BMI, CVD, diabetes, economic complications, functional limitations, gonadal axis, health status (perceived), body image, junction gastro-oesophagus, kidney disease, and liver disease (Table 2). For each domain, a person’s health is assigned a score of 0 (normal health), 1 (at risk), 2 (established disease) or 3 (advanced disease). The KOSC use quantifiable measures where possible to reduce the bias of subjective measures. A benefit of the King’s Criteria is that separate scores can be tracked for each domain, which allows for a more holistic and specific assessment of treatment benefit. So, patients with score 2 and 3 for each domain have increased all-cause mortality compared to stages 0 or 1 associated increase mortality rates (109).
Edmonton Obesity Staging System (EOSS)
The EOSS classifies the impact of obesity on an individual into five stages of severity (110). Stage 0 represents the obese phenotype with no co-morbidities. Stage 1 represents subclinical disease whereby medical management is favored. Patients in stages 2 and 3 of EOSS are candidates for medical and surgical intervention for obesity. Stage 4 represents end-stage disease and implies that surgical intervention is less likely to improve long-term prognosis and may be harmful (111).
Retrospective application of the EOSS to the National Health and Nutrition Examination Survey (NHANES) data showed that patients in stages 2–4 of EOSS have increased all-cause mortality compared to stages 0 or 1. The severity of the medical complication(s) had a far greater effect on survival than the BMI (111). The EOSS can also refine predictions of all-cause mortality within BMI categories of obesity.
Comparison of KOSC and EOSS
KOSC grades 12 domains of obesity-related morbidity arranged in an alphabetical manner each of them has scores 0–3, but EOSS classifies obese patients into five graded categories, based on their morbidity and health-risk profile. In KOSC separate scores can be tracked for each domain which allows for a more holistic and specific assessment for the risk of complications and mortality, which is not applicable in EOSS. The EOSS provides prognostic information that can assist clinicians in tailoring interventions in a simple manner; Stage 0 no need for treatment, Stage 1 represents subclinical disease whereby medical management is favored. Patients in stages 2 and 3 of EOSS are candidates for medical and surgical intervention for obesity. Stage 4 represents end-stage disease and implies that surgical intervention is the only feasible remaining option. However in KOSC tailoring of intervention is more complicated.
Weight loss impact on morbidity and mortality
The life expectancy of a severely obese person is reduced by an estimated 5–20 years (112). A large cohort prospective study and other retrospective cohort studies suggested that bariatric surgery reduces mortality considerably. In the Swedish Obese Subjects (SOS) study, during a period of up to 15 years, the overall mortality was 30.7% lower among the bariatric group compared with control subjects, and the most common causes of death were myocardial infarction and cancer with much of surgery-induced reductions in the latter accounted for by diminished incidence of female cancers, particularly endometrial cancer (113).
In a large retrospective cohort study, during a mean follow-up of 7.1 years, adjusted long-term mortality from any cause in the surgery group decreased by 40% compared with the control group. The cause-specific mortality rate in the surgery group decreased by 56% for coronary artery disease, by 92% for diabetes, and by 60% for cancer (114).
Moreover, Flum and Dellinger reported a 33% reduction in the rate of death due to any cause after gastric bypass surgery as compared with the rate among control subjects after a mean follow-up of 4.4 years (115). Finally, Christou et al., at a mean follow-up of 2.6 years, reported that among patients who had undergone gastric bypass surgery, the rate of death due to any cause decreased by 89% compared with control subjects (116). So, weight loss associated with decrease morbidity and mortality.
Conclusions
The epidemic of obesity has highlighted the extent of the risks associated with this disease. The risks arise from the increased mass of fat tissue, as well as the products produced by the increased number and size of adipocytes in obese individuals. Psychosocial dysfunction, OSA, and osteoarthritis can be a direct result of increased fat mass. Other diseases associated with obesity result from the metabolic consequences of enlarged fat cells. Diabetes, gallbladder stones, high blood pressure, liver disease, coronary artery disease, cerebrovascular disease, certain types of cancers, and infertility can all be traced in part to the increased secretion of inflammatory and coagulation molecules from adipocytes. Finally, obesity also increases overall mortality. It is clear from this review that the morbidity and increased mortality of overweight and obesity are substantial and should prompt further attention towards the need for appropriate weight management in health care.
Acknowledgments
Funding: M Abdelaal was funded by a research award by the Ministry of Higher Education and Scientific Research of Egypt. Funding at the Dublin laboratory arises from a Science Foundation Ireland President of Ireland Young Researcher Award to CW le Roux (12/YI/B2480). CW le Roux (PI) and NG Docherty are co-investigators on a grant from the Swedish Research Council (Medicine and Health) (2015-02733) at University of Gothenburg.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Arroyo-Johnson C, Mincey KD. Obesity Epidemiology Worldwide. Gastroenterol Clin North Am 2016;45:571-9. [Crossref] [PubMed]
- Wang W, Wei PL, Lee YC, et al. Short-term results of laparoscopic mini-gastric bypass. Obes Surg 2005;15:648-54. [Crossref] [PubMed]
- Haslam DW, James WP. Obesity. Lancet 2005;366:1197-209. [Crossref] [PubMed]
- Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000;894:i-xii, 1-253. [PubMed]
- Meisinger C, Doring A, Thorand B, et al. Body fat distribution and risk of type 2 diabetes in the general population: are there differences between men and women? The MONICA/KORA Augsburg cohort study. Am J Clin Nutr 2006;84:483-9. [PubMed]
- Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006;444:881-7. [Crossref] [PubMed]
- Li S, Xiao J, Ji L, et al. BMI and waist circumference are associated with impaired glucose metabolism and type 2 diabetes in normal weight Chinese adults. J Diabetes Complications 2014;28:470-6. [Crossref] [PubMed]
- Misra A, Vikram NK, Gupta R, et al. Waist circumference cutoff points and action levels for Asian Indians for identification of abdominal obesity. Int J Obes (Lond) 2006;30:106-11. [Crossref] [PubMed]
- Visscher PM, Brown MA, McCarthy MI, et al. Five years of GWAS discovery. Am J Hum Genet 2012;90:7-24. [Crossref] [PubMed]
- Zaitlen N, Kraft P, Patterson N, et al. Using extended genealogy to estimate components of heritability for 23 quantitative and dichotomous traits. PLoS Genet 2013;9:e1003520. [Crossref] [PubMed]
- Zillikens MC, Yazdanpanah M, Pardo LM, et al. Sex-specific genetic effects influence variation in body composition. Diabetologia 2008;51:2233-41. [Crossref] [PubMed]
- Poveda A, Koivula RW, Ahmad S, et al. Innate biology versus lifestyle behaviour in the aetiology of obesity and type 2 diabetes: the GLACIER Study. Diabetologia 2016;59:462-71. [Crossref] [PubMed]
- Dixon JB. Obesity and diabetes: the impact of bariatric surgery on type-2 diabetes. World J Surg 2009;33:2014-21. [Crossref] [PubMed]
- Fetner R, McGinty J, Russell C, et al. Incretins, diabetes, and bariatric surgery: a review. Surg Obes Relat Dis 2005;1:589-97; discussion 97-8. [Crossref] [PubMed]
- Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world--a growing challenge. N Engl J Med 2007;356:213-5. [Crossref] [PubMed]
- Hartemink N, Boshuizen HC, Nagelkerke NJ, et al. Combining risk estimates from observational studies with different exposure cutpoints: a meta-analysis on body mass index and diabetes type 2. Am J Epidemiol 2006;163:1042-52. [Crossref] [PubMed]
- Xiao C, Giacca A, Lewis GF. Oral taurine but not N-acetylcysteine ameliorates NEFA-induced impairment in insulin sensitivity and beta cell function in obese and overweight, non-diabetic men. Diabetologia 2008;51:139-46. [Crossref] [PubMed]
- Yuzefovych L, Wilson G, Rachek L. Different effects of oleate vs. palmitate on mitochondrial function, apoptosis, and insulin signaling in L6 skeletal muscle cells: role of oxidative stress. Am J Physiol Endocrinol Metab 2010;299:E1096-105. [Crossref] [PubMed]
- Gurzov EN, Tran M, Fernandez-Rojo MA, et al. Hepatic oxidative stress promotes insulin-STAT-5 signaling and obesity by inactivating protein tyrosine phosphatase N2. Cell Metab 2014;20:85-102. [Crossref] [PubMed]
- Carmiel-Haggai M, Cederbaum AI, Nieto N. A high-fat diet leads to the progression of non-alcoholic fatty liver disease in obese rats. FASEB J 2005;19:136-8. [PubMed]
- Satapati S, Sunny NE, Kucejova B, et al. Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. J Lipid Res 2012;53:1080-92. [Crossref] [PubMed]
- Yokota T, Kinugawa S, Hirabayashi K, et al. Oxidative stress in skeletal muscle impairs mitochondrial respiration and limits exercise capacity in type 2 diabetic mice. Am J Physiol Heart Circ Physiol 2009;297:H1069-77. [Crossref] [PubMed]
- Riobo Servan P. Obesity and diabetes. Nutricion hospitalaria 2013;28 Suppl 5:138-43. [PubMed]
- Nakamura K, Fuster JJ, Walsh K. Adipokines: a link between obesity and cardiovascular disease. J Cardiol 2014;63:250-9. [Crossref] [PubMed]
- Grundy SM, Brewer HB Jr, Cleeman JI, et al. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol 2004;24:e13-8. [Crossref] [PubMed]
- Brassard P, Legault S, Garneau C, et al. Normalization of diastolic dysfunction in type 2 diabetics after exercise training. Med Sci Sports Exerc 2007;39:1896-901. [Crossref] [PubMed]
- Lakhani M, Fein S. Effects of obesity and subsequent weight reduction on left ventricular function. Cardiol Rev 2011;19:1-4. [Crossref] [PubMed]
- Wanahita N, Messerli FH, Bangalore S, et al. Atrial fibrillation and obesity--results of a meta-analysis. Am Heart J 2008;155:310-5. [Crossref] [PubMed]
- Kotchen TA, Grim CE, Kotchen JM, et al. Altered relationship of blood pressure to adiposity in hypertension. Am J Hypertens 2008;21:284-9. [Crossref] [PubMed]
- Flegal KM KB, Orpana H, Graubard BI. Association of allcause mortality with overweight and obesity using standard body mass index categories: a systematic review and metaanalysis. JAMA 2013;309:71-82. [Crossref] [PubMed]
- Kaditis AG, Alexopoulos EI, Hatzi F, et al. Adiposity in relation to age as predictor of severity of sleep apnea in children with snoring. Sleep Breath 2008;12:25-31. [Crossref] [PubMed]
- Rudnick EF, Walsh JS, Hampton MC, et al. Prevalence and ethnicity of sleep-disordered breathing and obesity in children. Otolaryngol Head Neck Surg 2007;137:878-82. [Crossref] [PubMed]
- Kaw R, Hernandez AV, Walker E, et al. Determinants of hypercapnia in obese patients with obstructive sleep apnea: a systematic review and metaanalysis of cohort studies. Chest 2009;136:787-96. [Crossref] [PubMed]
- Mokhlesi B, Saager L, Kaw R. Q. Should we routinely screen for hypercapnia in sleep apnea patients before elective noncardiac surgery? Cleve Clin J Med 2010;77:60-1. [Crossref] [PubMed]
- Mokhlesi B. Obesity hypoventilation syndrome: a state-of-the-art review. Respir Care 2010;55:1347-62; discussion 63-5. [PubMed]
- Mokhlesi B, Tulaimat A, Faibussowitsch I, et al. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep Breath 2007;11:117-24. [Crossref] [PubMed]
- Nowbar S, Burkart KM, Gonzales R, et al. Obesity-associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med 2004;116:1-7. [Crossref] [PubMed]
- Piper AJ, Grunstein RR. Obesity hypoventilation syndrome: mechanisms and management. Am J Respir Crit Care Med 2011;183:292-8. [Crossref] [PubMed]
- Akashiba T, Akahoshi T, Kawahara S, et al. Clinical characteristics of obesity-hypoventilation syndrome in Japan: a multi-center study. Intern Med 2006;45:1121-5. [Crossref] [PubMed]
- Campos-Rodriguez F, Pena-Grinan N, Reyes-Nunez N, et al. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest 2005;128:624-33. [Crossref] [PubMed]
- Priou P, Hamel JF, Person C, et al. Long-term outcome of noninvasive positive pressure ventilation for obesity hypoventilation syndrome. Chest 2010;138:84-90. [Crossref] [PubMed]
- Castro-Añón O, Pérez de Llano LA, De la Fuente Sánchez S, et al. Obesity-hypoventilation syndrome: increased risk of death over sleep apnea syndrome. PLoS One 2015;10:e0117808. [Crossref] [PubMed]
- Chinn S, Downs SH, Anto JM, et al. Incidence of asthma and net change in symptoms in relation to changes in obesity. Eur Respir J 2006;28:763-71. [Crossref] [PubMed]
- Beuther DA, Weiss ST, Sutherland ER. Obesity and asthma. Am J Respir Crit Care Med 2006;174:112-9. [Crossref] [PubMed]
- Abdeyrim A, Zhang Y, Li N, et al. Impact of obstructive sleep apnea on lung volumes and mechanical properties of the respiratory system in overweight and obese individuals. BMC Pulm Med 2015;15:76. [Crossref] [PubMed]
- Littleton SW. Impact of obesity on respiratory function. Respirology 2012;17:43-9. [Crossref] [PubMed]
- Soylemezoglu O, Duzova A, Yalcinkaya F, et al. Chronic renal disease in children aged 5-18 years: a population-based survey in Turkey, the CREDIT-C study. Nephrol Dial Transplant 2012;27 Suppl 3:iii146-51. [Crossref] [PubMed]
- Tsuboi N, Utsunomiya Y, Kanzaki G, et al. Low glomerular density with glomerulomegaly in obesity-related glomerulopathy. Clin J Am Soc Nephrol 2012;7:735-41. [Crossref] [PubMed]
- Gilardini L, Zulian A, Girola A, et al. Predictors of the early impairment of renal disease in human obesity. Int J Obes (Lond) 2010;34:287-94. [Crossref] [PubMed]
- Liese AD, Hense HW, Doring A, et al. Microalbuminuria, central adiposity and hypertension in the non-diabetic urban population of the MONICA Augsburg survey 1994/95. J Hum Hypertens 2001;15:799-804. [Crossref] [PubMed]
- Bello AK, de Zeeuw D, El Nahas M, et al. Impact of weight change on albuminuria in the general population. Nephrol Dial Transplant 2007;22:1619-27. [Crossref] [PubMed]
- Prospective Studies C, Whitlock G, Lewington S, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373:1083-96. [Crossref] [PubMed]
- Ruan XZ, Varghese Z, Moorhead JF. An update on the lipid nephrotoxicity hypothesis. Nat Rev Nephrol 2009;5:713-21. [Crossref] [PubMed]
- Goek ON, Kottgen A, Hoogeveen RC, et al. Association of apolipoprotein A1 and B with kidney function and chronic kidney disease in two multiethnic population samples. Nephrol Dial Transplant 2012;27:2839-47. [Crossref] [PubMed]
- Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 2013;10:330-44. [Crossref] [PubMed]
- Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 2013;10:686-90. [Crossref] [PubMed]
- Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol 2006;45:600-6. [Crossref] [PubMed]
- Li Z, Clark J, Diehl AM. The liver in obesity and type 2 diabetes mellitus. Clin Liver Dis 2002;6:867-77. [Crossref] [PubMed]
- Argo CK, Northup PG, Al-Osaimi AM, et al. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol 2009;51:371-9. [Crossref] [PubMed]
- Ascha MS, Hanouneh IA, Lopez R, et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010;51:1972-8. [Crossref] [PubMed]
- Ratziu V, Bonyhay L, Di Martino V, et al. Survival, liver failure, and hepatocellular carcinoma in obesity-related cryptogenic cirrhosis. Hepatology 2002;35:1485-93. [Crossref] [PubMed]
- Bugianesi E, Leone N, Vanni E, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology 2002;123:134-40. [Crossref] [PubMed]
- Hashimoto K, Hirai M, Kurosawa Y. Identification of a mouse homolog for the human hereditary haemochromatosis candidate gene. Biochem Biophys Res Commun 1997;230:35-9. [Crossref] [PubMed]
- Pasquali R, Patton L, Gambineri A. Obesity and infertility. Curr Opin Endocrinol Diabetes Obes 2007;14:482-7. [Crossref] [PubMed]
- Gosman GG, King WC, Schrope B, et al. Reproductive health of women electing bariatric surgery. Fertil Steril 2010;94:1426-31. [Crossref] [PubMed]
- Levens ED, Skarulis MC. Assessing the role of endometrial alteration among obese patients undergoing assisted reproduction. Fertil Steril 2008;89:1606-8. [Crossref] [PubMed]
- Gosman GG, Katcher HI, Legro RS. Obesity and the role of gut and adipose hormones in female reproduction. Hum Reprod Update 2006;12:585-601. [Crossref] [PubMed]
- Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013;108:308-28. [Crossref] [PubMed]
- Lee YY, McColl KE. Pathophysiology of gastroesophageal reflux disease. Best Pract Res Clin Gastroenterol 2013;27:339-51. [Crossref] [PubMed]
- Pandolfino JE, Kwiatek MA, Kahrilas PJ. The pathophysiologic basis for epidemiologic trends in gastroesophageal reflux disease. Gastroenterol Clin North Am 2008;37:827-43. viii. [Crossref] [PubMed]
- El-Serag HB, Ergun GA, Pandolfino J, et al. Obesity increases oesophageal acid exposure. Gut 2007;56:749-55. [Crossref] [PubMed]
- Hampel H, Abraham NS, El-Serag HB. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med 2005;143:199-211. [Crossref] [PubMed]
- Nomura DK, Long JZ, Niessen S, et al. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell 2010;140:49-61. [Crossref] [PubMed]
- Wymann MP, Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol 2008;9:162-76. [Crossref] [PubMed]
- Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet 2010;375:2267-77. [Crossref] [PubMed]
- Mu N, Zhu Y, Wang Y, et al. Insulin resistance: a significant risk factor of endometrial cancer. Gynecol Oncol 2012;125:751-7. [Crossref] [PubMed]
- Lukanova A, Lundin E, Zeleniuch-Jacquotte A, et al. Body mass index, circulating levels of sex-steroid hormones, IGF-I and IGF-binding protein-3: a cross-sectional study in healthy women. Eur J Endocrinol 2004;150:161-71. [Crossref] [PubMed]
- Bezemer ID, Rinaldi S, Dossus L, et al. C-peptide, IGF-I, sex-steroid hormones and adiposity: a cross-sectional study in healthy women within the European Prospective Investigation into Cancer and Nutrition (EPIC). Cancer Causes Control 2005;16:561-72. [Crossref] [PubMed]
- Santen RJ, Boyd NF, Chlebowski RT, et al. Critical assessment of new risk factors for breast cancer: considerations for development of an improved risk prediction model. Endocr Relat Cancer 2007;14:169-87. [Crossref] [PubMed]
- Marmot M, Atinmo T, Byers T, et al. editors. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington DC: AICR, 2007.
- Munsell MF, Sprague BL, Berry DA, et al. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev 2014;36:114-36. [Crossref] [PubMed]
- Gaudet MM, Press MF, Haile RW, et al. Risk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or younger. Breast Cancer Res Treat 2011;130:587-97. [Crossref] [PubMed]
- Chan DS, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol 2014;25:1901-14. [Crossref] [PubMed]
- Niraula S, Ocana A, Ennis M, et al. Body size and breast cancer prognosis in relation to hormone receptor and menopausal status: a meta-analysis. Breast Cancer Res Treat 2012;134:769-81. [Crossref] [PubMed]
- Azrad M, Demark-Wahnefried W. The association between adiposity and breast cancer recurrence and survival: A review of the recent literature. Curr Nutr Rep 2014;3:9-15. [Crossref] [PubMed]
- Jiralerspong S, Kim ES, Dong W, et al. Obesity, diabetes, and survival outcomes in a large cohort of early-stage breast cancer patients. Ann Oncol 2013;24:2506-14. [Crossref] [PubMed]
- Tait S, Pacheco JM, Gao F, et al. Body mass index, diabetes, and triple-negative breast cancer prognosis. Breast Cancer Res Treat 2014;146:189-97. [Crossref] [PubMed]
- Bardel A, Wallander MA, Svardsudd K. Reported current use of prescription drugs and some of its determinants among 35 to 65-year-old women in mid-Sweden: A population-based study. J Clin Epidemiol 2000;53:637-43. [Crossref] [PubMed]
- Narbro K, Agren G, Jonsson E, et al. Pharmaceutical costs in obese individuals: comparison with a randomly selected population sample and long-term changes after conventional and surgical treatment: the SOS intervention study. Arch Intern Med 2002;162:2061-9. [Crossref] [PubMed]
- Ghobadi C, Johnson TN, Aarabi M, et al. Application of a systems approach to the bottom-up assessment of pharmacokinetics in obese patients: expected variations in clearance. Clin Pharmacokinet 2011;50:809-22. [Crossref] [PubMed]
- Wadden TA, Sarwer DB, Fabricatore AN, et al. Psychosocial and behavioral status of patients undergoing bariatric surgery: what to expect before and after surgery. Med Clin North Am 2007;91:451-69. xi-xii. [Crossref] [PubMed]
- Al-Agha AE, Al-Ghamdi RA, Halabi SA. Correlation between obesity and emotional, social, and behavioral problems associated with physical limitation among children and adolescents in Western Saudi Arabia. Saudi Med J 2016;37:161-5. [Crossref] [PubMed]
- Peltonen M, Lindroos AK, Torgerson JS. Musculoskeletal pain in the obese: a comparison with a general population and long-term changes after conventional and surgical obesity treatment. Pain 2003;104:549-57. [Crossref] [PubMed]
- Jiménez Buñuales MT, González Diego P, Martín Moreno JM, et al. International classification of functioning, disability and health (ICF) 2001. Rev Esp Salud Publica 2002;76:271-9. [Crossref] [PubMed]
- Capodaglio P, Cimolin V, Tacchini E, et al. Effectiveness of in-patient rehabilitation in obesity-related orthopedic conditions. J Endocrinol Invest 2013;36:628-31. [PubMed]
- Wearing SC, Hennig EM, Byrne NM, et al. The biomechanics of restricted movement in adult obesity. Obes Rev 2006;7:13-24. [Crossref] [PubMed]
- WHO 2016. Available online: http://www.euro.who.int/HEN/Syntheses/short/20040908_1
- Williams GA, Hudson DL, Whisenhunt BL, et al. An examination of body tracing among women with high body dissatisfaction. Body Image 2014;11:346-9. [Crossref] [PubMed]
- Slade PD. What is body image? Behav Res Ther 1994;32:497-502. [Crossref] [PubMed]
- Dietz WH. Health consequences of obesity in youth: childhood predictors of adult disease. Pediatrics 1998;101:518-25. [PubMed]
- Trull TJ, Verges A, Wood PK, et al. The structure of Diagnostic and Statistical Manual of Mental Disorders (4th edition, text revision) personality disorder symptoms in a large national sample. Personal Disord 2012;3:355-69.
- Lehnert T, Sonntag D, Konnopka A, et al. Economic costs of overweight and obesity. Best Pract Res Clin Endocrinol Metab 2013;27:105-15. [Crossref] [PubMed]
- Lang T, Rayner G, Rayner M, et al. Policy councils on food, nutrition and physical activity: the UK as a case study. Public Health Nutr 2005;8:11-9. [Crossref] [PubMed]
- Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med 2010;363:2211-9. [Crossref] [PubMed]
- Romero-Corral A, Montori VM, Somers VK, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet 2006;368:666-78. [Crossref] [PubMed]
- Flegal KM, Graubard BI, Williamson DF, et al. Excess deaths associated with underweight, overweight, and obesity. JAMA 2005;293:1861-7. [Crossref] [PubMed]
- Manson JE, Bassuk SS, Hu FB, et al. Estimating the number of deaths due to obesity: can the divergent findings be reconciled? J Womens Health (Larchmt) 2007;16:168-76. [Crossref] [PubMed]
- Flegal KM, Kit BK, Orpana H, et al. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA 2013;309:71-82. [Crossref] [PubMed]
- Aylwin S, Al-Zaman Y. Emerging concepts in the medical and surgical treatment of obesity. Front Horm Res 2008;36:229-59. [Crossref] [PubMed]
- Padwal RS, Pajewski NM, Allison DB, et al. Using the Edmonton obesity staging system to predict mortality in a population-representative cohort of people with overweight and obesity. CMAJ 2011;183:E1059-66. [Crossref] [PubMed]
- Kuk JL, Ardern CI, Church TS, et al. Edmonton Obesity Staging System: association with weight history and mortality risk. Appl Physiol Nutr Metab 2011;36:570-6. [Crossref] [PubMed]
- Fontaine KR, Redden DT, Wang C, et al. Years of life lost due to obesity. JAMA 2003;289:187-93. [Crossref] [PubMed]
- jostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741-52.
- Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med 2007;357:753-61. [Crossref] [PubMed]
- Flum DR, Dellinger EP. Impact of gastric bypass operation on survival: a population-based analysis. J Am Coll Surg 2004;199:543-51. [Crossref] [PubMed]
- Christou NV, Sampalis JS, Liberman M, et al. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg 2004;240:416-23; discussion 23-4. [Crossref] [PubMed]