Alpha-interferon treatment in hepatitis B
Introduction
Interferons are cytokines, a large family of low molecular weight (15–30 kDa), soluble glycoproteins with potent antiviral activities (1,2). Interferon-α (IFN-α) is produced by the plasmacytoid dendritic cells (3) and its use for treating hepatitis B dates back to 1976 (4). Subsequently, a thrice weekly treatment regimen using human lymphoblastoid interferon was adopted (5).
The emergence of pegylated interferon-α (PEG-IFN-α) in 2005 resulted in standard interferon being replaced. The pegylation of IFN-α improves the pharmacokinetics and prolongs drug half-life, allowing for once a week subcutaneous injections. The two major forms of PEG-IFN are PEG-IFN-α2a (Pegasys® Roche) and PEG-IFN-α2b (Pegintron® Merck). A 24-week treatment course of once a week PEG-IFN-α2a (Pegasys) resulted in a higher rate of response of 24% versus 12% in those using standard IFN-α, defined as Hepatitis B e Antigen (HBeAg) loss, hepatitis B virus DNA (HBV DNA) less than half million copies per mL, and ALT normalization (6). A comparison study of Chinese patients treated for 24 weeks with PEG-IFN-α2b (Pegintron) demonstrated a greater HBeAg loss compared to those who were treated with standard IFN-α (24.4% vs. 13.9%) (7). However, both studies used IFN-α doses that are lower than the recommended 5–10 million units thrice weekly (8), and so superiority of PEG-IFN-α over standard IFN-α in terms of treatment efficacy alone is contentious. The use of PEG-IFN-α has superseded standard IFN-α mostly because of a more convenient dosing regimen which has resulted in improved patient compliance and acceptability.
Mode of action
The exact mechanism of how interferon affects hepatitis B is unknown. It thought to act on various parts of the HBV lifecycle as well as augmenting cell mediated immunity. IFN-α inhibits HBV replication by decreasing RNA transcription, occurring from covalently closed circular DNA (cccDNA) (9). Interferon results in cccDNA-bound histone hypoacetylation and cccDNA transcriptional co-repressor active recruitment.
Apobec3G (apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like 3G) expression in hepatitis B patients is lower compared with non-infected controls. Apobec3G induces G to A hypermutation in hepatitis B viral DNA, which strongly inhibits replication. Apobec3G may also inhibit the HBV lifecycle by interacting with HBV core protein. IFN induces Apobec3G protein expression, in association with STAT3 activation. Hepatitis B surface antigen (HBsAg) has been found to inhibit IFN induced Apobec3G up-regulation in a dose dependent manner (10).
The immune response from CD8 T cells and NK cells in hepatitis B is dysfunctional. PEG-IFN-α has been reported to cumulatively drive proliferation, activation and antiviral potential of NK cells (11). A restoration of NK cell responses is associated with a greater decline in HBsAg when patients are given PEG-IFN-α (12).
Pegylated interferon alpha versus nucleos(t)ide analogues (NAs)
PEG-IFN and NAs are the main forms of antiviral treatment for CHB. NAs were developed during the 1980s for the treatment of HIV, but subsequently were found to have additional efficacy in treating CHB. There are advantages and disadvantages of therapy with PEG-IFN compared to NAs. PEG-IFN treatment has the benefit of finite treatment duration, a higher rate of HBeAg and HBsAg seroconversion, a higher chance of sustained off-treatment response, and no drug resistance (13). On the other hand, PEG-IFN therapy is not well tolerated because adverse effects are common and can occasionally cause significant morbidity or mortality. Pregnancy and decompensated cirrhosis are absolute contraindications. Administration by subcutaneous injection is difficult for some patients. The advantages of NAs are that it is an oral medication, is a potent anti-viral, and has relatively few adverse effects. NAs are safe to use in cirrhosis and some are safe in pregnancy. The newer NAs, entecavir monohydrate (ETV) and tenofovir disoproxil fumarate (TDF) also have little or no drug resistance (14,15). The main disadvantage of NAs are that rates of HBeAg and HBsAg seroconversion are lower, and sustained off-treatment responses are rare (13). As a result, the treatment duration is usually indefinite.
The relative advantages of using PEG-IFN therapy for CHB must be carefully measured against its disadvantages for each individual. The appropriateness of treatment often depends on considering the likelihood of achieving a sustained off-treatment response against the greater adverse effects. The factors that determine a sustained response are different for HBeAg positive and HBeAg negative disease, and can be broadly categorized into pre-treatment factors, on-treatment factors, dosing and duration of therapy. As PEG-IFN and NAs have different mechanisms of action, it has been hypothesized that combining the two drug classes could improve rates of cure. These issues are discussed in the following sections.
HBeAg positive disease
Pre-treatment factors for sustained response
The efficacy of PEG-IFN monotherapy in HBeAg positive patients was established in the two registration studies (16,17). Treatment with PEG-IFN-α2a or PEG-IFN-α2b for 1 year resulted in HBeAg seroconversion in 22–27% at the end of treatment and 29–32% at 6 months post treatment. Off-treatment viral suppression (defined as HBV DNA <400 copies/mL) was achieved in 7–14%, and HBsAg seroconversion occurred in 3–5% of patients (16,17).
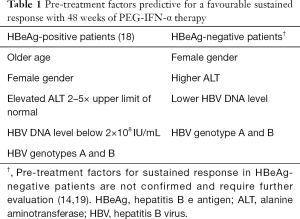
The importance of HBV genotypes and other parameters in the responsiveness to IFN was demonstrated in another study which pooled data from the two registration trials (18). Baseline predictors of a favourable response include HBV genotypes A and B, lower HBV DNA level (below 2×108 IU/mL), high ALT levels that are 2–5× upper limit of normal, older age and female gender (18) (see Table 1). Based on these variables, a multivariate model was developed—the so-called PEG-IFN treatment index. This provides general recommendations to consider PEG-IFN therapy when the predicted probability of durable response is greater than 30% (13).
Full table
In CHB patients with severe liver disease, PEG-IFN is safe to use in advanced fibrosis and compensated cirrhosis. A study by Buster et al. reported that adverse events were observed equally in those with advanced fibrosis compared to those without. Furthermore, the virologic response was observed to be higher (30% vs. 14%) in the small subgroup of cirrhotic patients (n=24), compared to those without (20).
On-treatment factors for sustained response
Treatment failure can be predicted based on the quantitative level of HBsAg during therapy, but out of the three major regional guidelines, only the European Association for the Study of the Liver (EASL) provide suggestions for when to stop therapy. Cessation of PEG-IFN is recommended if HBsAg levels are above 20,000 IU/mL or if no decline occurs at 12 weeks in comparison with baseline levels (14). These recommendations are based on findings by Sonneveld et al., who reported that no decline in HBsAg levels at week 12 is associated with a negative predictive value (NPV) of 97% for a sustained viral response and 100% NPV for HBsAg loss (21). These findings were supported by the NEPTUNE phase 3 study, in which no patients experienced sustained HBeAg seroconversion (defined as 6 months post treatment) if the HBsAg level was >20,000 IU/mL at week 12 of therapy (22,23).
Although on-treatment HBsAg quantification can predict treatment failure, it is not as reliable in predicting treatment success. In the NEPTUNE study, the best positive predictive values (PPV) for sustained HBeAg seroconversion was 57% and 54%, which occurred when HBsAg levels ≤1,500 IU/mL at 12 and 24 weeks of therapy respectively. The corresponding NPVs were 72% and 76% respectively (22,23). In a study by Chan et al. (24), the highest predictive scores were PPV 75% and NPV 85%, which occurred in patients who had both HBsAg ≤300 IU/mL along with HBsAg decrease of ≥1 log10 IU/mL at 24 weeks compared to baseline. Table 2 provides a summary of on treatment factors predicting treatment success or failure with PEG-IFN in HBeAg positive disease.

Full table
Quantitative HBeAg levels may be helpful in predicting sustained response, but studies are few. A significant reduction in HBeAg levels were reported in all treatment groups in a trial comparing combination Peg-IFN-α2a plus ETV against Peg-IFN-α2a monotherapy (29). In another study that evaluated sequential combination therapy of PEG-IFN plus ETV, a baseline level HBeAg <200 signal-to-cut-off ratio, along with a baseline HBsAg of <1,000 IU/mL and a HBsAg decline at week 12 of ≥0.5 log10 IU/mL, was associated with week 48 HBeAg seroconversion rate of 92% and HBsAg loss of 83.3% (30). Further studies are required to determine the value of quantitative HBeAg in predicting an off-treatment sustained response and its role in monitoring during treatment.
Dose and duration of treatment
Current recommended treatment with PEG-IFN is for a duration of 48 weeks at a dose of 180 µg per week (14,19,31). Data for this approach is based largely on the 4-arm NEPTUNE study which aimed to provide a consensus on dosing and duration by comparing 90 vs. 180 µg weekly, and 24 vs. 48 weeks PEG-IFN-α2a treatment (32). The highest HBeAg seroconversion rate (36.2%) at 6 months post treatment, was achieved in those who received a dose of 180 µg weekly for 48 weeks, followed by 90 µg weekly for 48 weeks (25.8%), 180 µg weekly for 24 weeks (22.9%) and 90 µg weekly for 24 weeks (14.1%) (32).
Combination and sequential treatment with NAs
Many early studies combining lamivudine (LAM) and PEG-IFN failed to show any convincing evidence of benefit. Several recent studies have explored the use of combination and in sequential therapy with the latest generation NAs therapy, ETV and TDF.
Comparison of PEG-IFN plus LAM against PEG-IFN or LAM alone was performed in three major studies (16,17,33). In the registration trial of PEG-IFN-α2a, three treatment arms: PEG-IFN plus LAM, PEG-IFN monotherapy or LAM monotherapy for 48 weeks were compared in 814 patients (17). At week 72, there was no significant difference in viral suppression, defined as DNA <100,000 copies/mL (34% vs. 32%), and HBeAg seroconversion (27% vs. 32%) in the combination therapy group compared with the PEG-IFN monotherapy group. However, the LAM monotherapy group demonstrated lower rates of viral suppression (22%) and HBeAg seroconversion (19%) (17). In the HBV 99-01 European multicentre study, combination therapy with PEG-IFN-α2b plus LAM was compared against PEG-IFN-α2b monotherapy in 307 patients for 52 weeks (16). Post treatment at week 78, there was no difference in HBeAg seroclearance (35% vs. 36%), HBV DNA suppression, defined as <400 copies/mL (9% vs. 7%), HBsAg loss (both 7%) or ALT normalization (35% vs. 32%) (16). The third study compared treatment with 52 weeks of sequential combined therapy with PEG-IFN-α2b plus LAM (PEG-IFN and LAM was given combined for the first 32 weeks, followed by LAM monotherapy for the remaining period), against 52 weeks of LAM monotherapy (33). At post treatment week 24, viral suppression (defined as HBV DNA <500,000 copies/mL) and HBeAg seroconversion occurred in 36% in the combination group versus 14% in the LAM monotherapy group. Overall, these three studies showed that PEG-IFN, whether in combination with LAM or as PEG-IFN monotherapy, achieves a higher rate of sustained HBeAg seroconversion and greater viral suppression than LAM monotherapy. However, the combination of PEG-IFN and LAM was not demonstrated to have a higher sustained response over PEG-IFN alone.
A reason for the lack of a superior response from combination therapy may be that a longer treatment duration is required. The effectiveness of extended treatment was investigated in a study conducted in China, where 47 patients were treated with 96 weeks of PEG-IFN-α2a in combination with LAM or adefovir disoproxil (ADV). At 6 months post treatment, this strategy achieved high rates of HBeAg and HBsAg seroconversion of 72.3% and 27.7%, respectively. The results should be interpreted with caution as this was a small study with no PEG-IFN monotherapy arm for comparison (34).
In the study of telbivudine plus PEG-IFN-α2a, a rapid and profound reduction in HBV DNA levels was reported (35). However, the combination of PEG-IFN and telbivudine was found to be associated with an increased risk of peripheral neuropathy, which resulted in early trial termination. Thus this combination is not recommended.
Studies of ETV in combination with PEG-IFN have been performed, but none so far have been conclusive. In a study conducted in China, 218 treatment-naive HBeAg-positive patients were randomized to either 48 weeks monotherapy with PEG-IFN-α2a, 48 weeks of PEG-IFN-α2a combined intercurrent with 24weeks of ETV (ETV added at week 13 and continued for 24 weeks), or 48 weeks of PEG-IFN-α2a in sequence after a 24-week pre-treatment course of ETV (29). At 6 months post treatment, there was no significant difference in HBeAg seroconversion (25–31%), HBV DNA <1,000 copies/mL (23–29%), ALT normalisation (26–32%) or HBsAg seroconversion (1.4–4.1%) across all three groups (29). In the ARES study, 175 HBeAg positive CHB patients started on ETV monotherapy and were randomized to either 24 weeks of PEG-IFN add-on therapy (started at week 24 and given until week 48), or to continue ETV monotherapy (36). At week 96, the rate of HBeAg loss and viral suppression (defined as HBV DNA <200 IU/mL) was greater, but not statistically significant in the add-on therapy arm compared to the monotherapy arm (19% vs. 10%, P=0.095) (36). The study also lacked a PEG-IFN monotherapy arm for comparison.
A number of studies have investigated the use of PEG-IFN after long term therapy with NAs, where it has been theorized that higher rates of durable response may occur if viral suppression has already been achieved. In 197 patients who had at least 2 years prior experience with ETV, sequential treatment with combination PEG-IFN-α2a plus ETV was compared against continued ETV monotherapy for 48 weeks (30). At week 96, the sequential combination therapy group achieved greater HBeAg seroconversion than those in the ETV group (44% versus 6%), but HBsAg loss occurred only in two patients belonging to the combination group, and was not statistically significant (30). In the OSST trial, 197 patients on long-term ETV were randomised 1:1 to be switched to receive PEG-IFN-α2a alone, or continue ETV for 48 weeks (37). At week 48, HBeAg seroconversion occurred in 14.9% and 6.1% in the PEG-IFN and ETV arms respectively. HBsAg loss occurred only in the PEG-IFN arm at 8.5% (37). A similar study conducted in Korea, the Roll Over trial, examined the effect of switching to PEG-IFN in patients who had been treated with not only ETV, but any prior NAs, and who have an undetectable HBV DNA (<80 IU/mL) for at least 1 year (38). A report of their interim analysis at 48 weeks, reveals that on treatment HBsAg decline (log100.302 vs. log100.014) and HBeAg seroconversion (26% vs. 0%) were significantly higher in patients who had been switched to receive PEG-IFN-α2a than those who continued NAs (38). Lastly, switching to extended duration PEG-IFN therapy was examined in patients treated with prior long term NAs in an open label phase IV, randomized multicenter study in China (NEW SWITCH study) (39). A total of 303 HBeAg-positive CHB patients on NAs (ADV, LAM or ETV) for 1–3 years and who already had viral suppression (defined as HBV <200 IU/mL) and HBeAg loss were recruited. Treatment was randomized to be either 48 or 96 weeks PEG-IFN-α2a, with the first 12 weeks overlapping with their current NAs therapy. HBeAg seroconversion at 1-year post treatment occurred in 43.1% and 49.3% in the 48 and 96 weeks treatment groups respectively, while sustained HBsAg loss occurred in 9.2% and 13.3% respectively (39). In summary, sequential switching to a finite duration of PEG-IFN appears to enable higher sustained responses in HBeAg seroconversion and HBsAg loss compared to those who continue monotherapy with NAs. However, since all these studies sought to evaluate sequential treatment to patients already on NA therapy, the question of whether similar results could have been achieved with PEG-IFN alone on a treatment naïve patient from the very beginning cannot be resolved.
Recently, study 149 investigators reported promising results for combination TDF and PEG-IFN therapy in their multicentre open-label active-controlled study of 740 CHB patients. In those who received TDF plus PEG-IFN for 48 weeks experienced a greater rate of HBsAg loss at week 72 (9.1%) compared to those who received 16 weeks of TDF plus PEG-IFN followed by 32 weeks of TDF only (2.8%), TDF only for 120 weeks (0%) and PEG-IFN only for 48 weeks (2.8%) (40). This study is amongst the first to provide substantial evidence of patients receiving a potent oral antiviral agent with a high barrier to resistance, can achieve higher rates of HBsAg loss in combination with PEG-IFN, compared to PEG-IFN monotherapy. It also lends support to the concept of combination therapy of finite duration for patients with CHB. Further studies are required to identify the optimal combination therapy regimen and subgroups with the highest likelihood of response.
HBeAg negative disease
Pre-treatment factors for sustained response
Factors associated with sustained off treatment response in HBeAg negative disease are less well defined compared to HBeAg positive disease (14,19) (see Table 1). Similar parameters such as genotype A, female gender, high ALT and low HBV DNA loads may be associated, but further research is needed. A meta-analysis concluded that genotype B is more responsive compared with genotype C to PEG-IFN treatment (41).
On treatment factors for sustained response
Quantitative HBsAg and HBV DNA levels have been demonstrated to be useful in predicting a poor response. Of the three major regional guidelines, only EASL provide recommendations. Cessation of PEG-IFN therapy in HBeAg negative patients is suggested when there is a lack of HBsAg decline combined with less than 2 log10 IU/mL decline of HBV DNA at week 12 of therapy compared to baseline—known as the PARC stopping rule (14). In the 107 patients from the original PARC trial, these parameters were demonstrated to have 100% NPV (42). The PARC study had patients who had genotypes A and D, but none who had genotypes B and C. Hence the PARC stopping rule was separately validated in a retrospective study of 160 patients derived from two different cohorts that also included genotypes B and C (26). The stopping rule was reported to have 95% NPV for all genotypes, and 100% for genotype D. Furthermore, the PARC rule performed well regardless of whether PEG-IFN-α2a was given for 48 or 96 weeks (26). However, the rule would only identify 20% of the patients from the original PARC trial who achieved a sustained response and 14% of patients from the validation cohorts. A Greek study found that HBsAg decline <10% at 24 weeks compared to baseline was associated with 90% NPV (27). When combined with the PARC rule, 67% of treatment failures could be identified. However, the study was small (n=95) and the combination stopping rule was developed from an even smaller subset (n=47) due to a lack of complete data. Further validation is required.
Predictors of treatment success with PEG-IFN in HBeAg negative patients are not well established. In 518 patients from the original phase III trial (28), the best PPV was 50%, which was associated with HBV DNA decrease to less than 20,000 copies/mL at 12 weeks (43). Although HBsAg levels correlate with sustained viral suppression (25) its use as a predictor is poor. In the aforementioned Greek study, the best PPV was 45% which occurred when HBsAg decline was greater than 10% at 24 weeks (27). Table 2 provides a summary of on treatment factors predicting treatment success or failure with PEG-IFN in HBeAg negative disease.
Treatment duration and dose
The dose and duration of PEG-IFN is recommended to be 180 µg weekly for 48 weeks for both HBeAg negative and HBeAg disease (14,19,31). In the phase III registration trial of HBeAg negative patients, 48 weeks of treatment with PEG-IFN-α2a resulted in a sustained off-treatment virological response (defined as HBV DNA <400 copies/mL at 6 months post treatment) of 20% and HBsAg loss 3% (28). In the long term follow up study, 23% of patients had persistent viral suppression (defined as HBV DNA <2,000 IU/mL) after 5 years (44). In addition, the rate of HBsAg loss increased to 9% at 3 years and 12% at 5 years (45,46). It was noted that higher rates of HBsAg clearance (28%) at 5 years post-treatment occurred when patients had HBV DNA less than 2,000 IU/mL at 1-year post-treatment (44).
Extended therapy in HBeAg negative patients was explored in an Italian multicentre centre study (47), which compared 128 mostly genotype D patients (94%) randomised to 48 or 96 weeks of extended therapy with PEG-IFN-α2a (180 µg weekly for first 48 weeks for both groups, then 135 µg weekly in the extended therapy arm only). Significantly higher sustained off-treatment viral suppression, defined as HBV DNA <2,000 IU/mL at 12 months post treatment (29% vs. 12%), and HBsAg loss (6% vs. 0%) occurred in those in the 96 week extended therapy arm compared to the 48 weeks standard arm. The study reported that extended therapy was tolerated well, and that the rate of adverse events was comparable to 48 weeks treatment (48). Extended therapy with PEG-IFN may be an option, but needs further validation.
Combination and sequential treatment with NAs
Studies evaluating combination or sequential therapy for HBeAg negative disease are fewer compared to HBeAg positive disease. Early trials were unable to demonstrate any benefit. In the registration trial of PEG-IFN-α2a, no difference was observed in viral suppression, HBsAg loss, biochemical resolution nor histological improvement between patients receiving combination PEG-IFN plus LAM compared to PEG-IFN alone (28). Other early studies involving LAM or ADV and PEG-IFN also failed to show any superiority when using combination treatment (49,50).
More recent data have shown promising results. The PEGAN trial evaluated the addition of PEG-IFN for HBeAg negative patients with undetectable HBV DNA while on an established NAs dose for at least 12 months (51). Treatment with additional 48 weeks of PEG-IFN-α2a or no additional treatment was randomised in 183 patients. At week 96, HBsAg loss (11% vs. 3%) and HBsAg seroconversion (9% vs. 1%) were significantly greater than those with no additional treatment (51). In the previously mentioned Study 149 trial, the combination PEG-IFN and TDF therapy arm, in which HBsAg loss occurred in 9.1%, had almost 50% of participants who were HBeAg negative (40). HBsAg loss occurred in both HBeAg positive and HBeAg negative patients and across all major genotypes, with the highest rate occurring in genotype A. Further sub-analysis of HBeAg negative patients is awaited.
Conclusions
Sustained off-treatment response with PEG-IFN-α can be predicted by baseline factors in HBeAg positive disease, but not very well in HBeAg negative disease. Long term treatment failure can be identified using on-treatment quantitative HBsAg levels, but treatment success is not predicted reliably with any combination of on-treatment parameters. Up until recently, there was little evidence supporting the use of combination therapy with NAs. There are now promising results for the use of TDV in combination with PEG-IFN, and for the addition of PEG-IFN in patients who already have achieved viral suppression with other NAs therapy. In spite of this, cure remains elusive with rates of HBsAg loss reported to be in the order of only 10%. Further research is required to identify the optimal regimen of combination or sequential therapy, and which subgroups will have the highest likelihood of response.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci 1957;147:258-67. [Crossref] [PubMed]
- Isaacs A, Lindenmann J, Valentine RC. Pillars Article: Virus Interference. II. Some Properties of Interferon. Proc R Soc Lond B Biol Sci 1957;147:268-73. J Immunol 2015;195:1921-6. [PubMed]
- Reizis B, Bunin A, Ghosh HS, et al. Plasmacytoid dendritic cells: recent progress and open questions. Annu Rev Immunol 2011;29:163-83. [Crossref] [PubMed]
- Greenberg HB, Pollard RB, Lutwick LI, et al. Effect of human leukocyte interferon on hepatitis B virus infection in patients with chronic active hepatitis. N Engl J Med 1976;295:517-22. [Crossref] [PubMed]
- Lok AS, Weller IV, Karayiannis P, et al. Thrice weekly lymphoblastoid interferon is effective in inhibiting hepatitis B virus replication. Liver 1984;4:45-9. [Crossref] [PubMed]
- Cooksley WG, Piratvisuth T, Lee SD, et al. Peginterferon alpha-2a (40 kDa): an advance in the treatment of hepatitis B e antigen-positive chronic hepatitis B. J Viral Hepat 2003;10:298-305. [Crossref] [PubMed]
- Zhao H, Kurbanov F, Wan MB, et al. Genotype B and younger patient age associated with better response to low-dose therapy: a trial with pegylated/nonpegylated interferon-alpha-2b for hepatitis B e antigen-positive patients with chronic hepatitis B in China. Clin Infect Dis 2007;44:541-8. [Crossref] [PubMed]
- Hoofnagle JH, di Bisceglie AM. The treatment of chronic viral hepatitis. N Engl J Med 1997;336:347-56. [Crossref] [PubMed]
- Belloni L, Allweiss L, Guerrieri F, et al. IFN-α inhibits HBV transcription and replication in cell culture and in humanized mice by targeting the epigenetic regulation of the nuclear cccDNA minichromosome. J Clin Invest 2012;122:529-37. [Crossref] [PubMed]
- Xu F, Song H, Li N, et al. HBsAg blocks TYPE I IFN induced up-regulation of A3G through inhibition of STAT3. Biochem Biophys Res Commun 2016;473:219-23. [Crossref] [PubMed]
- Micco L, Peppa D, Loggi E, et al. Differential boosting of innate and adaptive antiviral responses during pegylated-interferon-alpha therapy of chronic hepatitis B. J Hepatol 2013;58:225-33. [Crossref] [PubMed]
- Gill US, Peppa D, Micco L, et al. Interferon Alpha Induces Sustained Changes in NK Cell Responsiveness to Hepatitis B Viral Load Suppression In Vivo. PLoS Pathog 2016;12:e1005788. [Crossref] [PubMed]
- Rijckborst V, Sonneveld MJ, Janssen HL. Review article: chronic hepatitis B - anti-viral or immunomodulatory therapy? Aliment Pharmacol Ther 2011;33:501-13. [Crossref] [PubMed]
- Ono A, Suzuki F, Kawamura Y, et al. Long-term continuous entecavir therapy in nucleos(t)ide-naïve chronic hepatitis B patients. J Hepatol 2012;57:508-14. [Crossref] [PubMed]
- Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet 2013;381:468-75. [Crossref] [PubMed]
- Janssen HL, van Zonneveld M, Senturk H, et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet 2005;365:123-9. [Crossref] [PubMed]
- Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med 2005;352:2682-95. [Crossref] [PubMed]
- Buster EH, Hansen BE, Lau GK, et al. Factors that predict response of patients with hepatitis Be antigen-positive chronic hepatitis B to peginterferon-alfa. Gastroenterology 2009;137:2002-9. [Crossref] [PubMed]
- Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016;63:261-83. [Crossref] [PubMed]
- Buster EH, Hansen BE, Buti M, et al. Peginterferon alpha-2b is safe and effective in HBeAg-positive chronic hepatitis B patients with advanced fibrosis. Hepatology 2007;46:388-94. [Crossref] [PubMed]
- Sonneveld MJ, Rijckborst V, Boucher CA, et al. Prediction of sustained response to peginterferon alfa-2b for hepatitis B e antigen-positive chronic hepatitis B using on-treatment hepatitis B surface antigen decline. Hepatology 2010;52:1251-7. [Crossref] [PubMed]
- Gane E, Jia J, Han K, et al. 69 Neptune Study: On-Treatment Hbsag Level Analysis Confirms Prediction of Response Observed in Phase 3 Study of Peginterferon Alfa-2a in Hbeag-Positive Patients. J Hepatol 2011;54:S31. [Crossref]
- Piratvisuth T, Marcellin P, Popescu M, et al. Hepatitis B surface antigen: association with sustained response to peginterferon alfa-2a in hepatitis B e antigen-positive patients. Hepatol Int 2013;7:429-36. [Crossref] [PubMed]
- Chan HL, Wong VW, Chim AM, et al. Serum HBsAg quantification to predict response to peginterferon therapy of e antigen positive chronic hepatitis B. Aliment Pharmacol Ther 2010;32:1323-31. [Crossref] [PubMed]
- Brunetto MR, Moriconi F, Bonino F, et al. Hepatitis B virus surface antigen levels: a guide to sustained response to peginterferon alfa-2a in HBeAg-negative chronic hepatitis B. Hepatology 2009;49:1141-50. [Crossref] [PubMed]
- Rijckborst V, Hansen BE, Ferenci P, et al. Validation of a stopping rule at week 12 using HBsAg and HBV DNA for HBeAg-negative patients treated with peginterferon alfa-2a. J Hepatol 2012;56:1006-11. [Crossref] [PubMed]
- Goulis I, Karatapanis S, Akriviadis E, et al. On-treatment prediction of sustained response to peginterferon alfa-2a for HBeAg-negative chronic hepatitis B patients. Liver Int 2015;35:1540-8. [Crossref] [PubMed]
- Marcellin P, Lau GK, Bonino F, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2004;351:1206-17. [Crossref] [PubMed]
- Xie Q, Zhou H, Bai X, et al. A randomized, open-label clinical study of combined pegylated interferon Alfa-2a (40KD) and entecavir treatment for hepatitis B “e” antigen-positive chronic hepatitis B. Clin Infect Dis 2014;59:1714-23. [Crossref] [PubMed]
- Li GJ, Yu YQ, Chen SL, et al. Sequential combination therapy with pegylated interferon leads to loss of hepatitis B surface antigen and hepatitis B e antigen (HBeAg) seroconversion in HBeAg-positive chronic hepatitis B patients receiving long-term entecavir treatment. Antimicrob Agents Chemother 2015;59:4121-8. [Crossref] [PubMed]
- Liaw YF, Kao JH, Piratvisuth T, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int 2012;6:531-61. [Crossref] [PubMed]
- Liaw YF, Jia JD, Chan HL, et al. Shorter durations and lower doses of peginterferon alfa-2a are associated with inferior hepatitis B e antigen seroconversion rates in hepatitis B virus genotypes B or C. Hepatology 2011;54:1591-9. [Crossref] [PubMed]
- Chan HL, Leung NW, Hui AY, et al. A randomized, controlled trial of combination therapy for chronic hepatitis B: comparing pegylated interferon-alpha2b and lamivudine with lamivudine alone. Ann Intern Med 2005;142:240-50. [Crossref] [PubMed]
- Cao ZH, Ma LN, Zhang HW, et al. Extended treatment with peginterferon α-2a in combination with lamivudine or adefovir for 96 weeks yields high rates of HBeAg and HBsAg seroconversion. J Dig Dis 2013;14:446-50. [Crossref] [PubMed]
- Marcellin P, Wursthorn K, Wedemeyer H, et al. Telbivudine plus pegylated interferon alfa-2a in a randomized study in chronic hepatitis B is associated with an unexpected high rate of peripheral neuropathy. J Hepatol 2015;62:41-7. [Crossref] [PubMed]
- Brouwer WP, Xie Q, Sonneveld MJ, et al. Adding pegylated interferon to entecavir for hepatitis B e antigen-positive chronic hepatitis B: A multicenter randomized trial (ARES study). Hepatology 2015;61:1512-22. [Crossref] [PubMed]
- Ning Q, Han M, Sun Y, et al. Switching from entecavir to PegIFN alfa-2a in patients with HBeAg-positive chronic hepatitis B: a randomised open-label trial (OSST trial). J Hepatol 2014;61:777-84. [Crossref] [PubMed]
- Woo HY, Heo J, Tak WY, et al. Change of HBsAg quantity and its relation with HBeAg seroconversion following 48 weeks pegylated-interferon-alpha treatment in HBeAg positive chronic hepatitis B patients after long term nucleos(t)ide analogue maintenance therapy (Roll Over trial): Interim analysis at 48 weeks. Hepatology 2016;64:1-136.
- Hu P, Dou X, Jiang J, et al. Increased and sustained HBsAg loss in HBeAg positive CHB patients switched from NUC to Peg-IFN alfa-2a: A randomised open label trial (NEW SWITCH study). Hepatology 2016;63:36A-7A.
- Marcellin P, Ahn SH, Ma X, et al. Combination of Tenofovir Disoproxil Fumarate and Peginterferon α-2a Increases Loss of Hepatitis B Surface Antigen in Patients With Chronic Hepatitis B. Gastroenterology 2016;150:134-44.e10. [Crossref] [PubMed]
- Kong LN, Qin B, Ma Q, et al. Relationship between hepatitis B virus genotype B and C and response to interferon therapy in HBeAg positive chronic hepatitis B patients: A meta-analysis. J Gastroenterol Hepatol 2014;29:1387-95. [Crossref] [PubMed]
- Rijckborst V, Hansen BE, Cakaloglu Y, et al. Early on-treatment prediction of response to peginterferon alfa-2a for HBeAg-negative chronic hepatitis B using HBsAg and HBV DNA levels. Hepatology 2010;52:454-61. [Crossref] [PubMed]
- Bonino F, Marcellin P, Lau GK, et al. Predicting response to peginterferon alpha-2a, lamivudine and the two combined for HBeAg-negative chronic hepatitis B. Gut 2007;56:699-705. [Crossref] [PubMed]
- Marcellin P, Bonino F, Yurdaydin C, et al. Hepatitis B surface antigen levels: association with 5-year response to peginterferon alfa-2a in hepatitis B e-antigen-negative patients. Hepatol Int 2013;7:88-97. [Crossref] [PubMed]
- Marcellin P, Bonino F, Lau GK, et al. Sustained response of hepatitis B e antigen-negative patients 3 years after treatment with peginterferon alpha-2a. Gastroenterology 2009;136:2169-79.e1-4.
- Marcellin P, Piratvisuth T, Brunetto M, et al. Increasing rates of HBsAg clearance and seroconversion in patients with HBeAg-Negative disease treated with peginterferon ALFA-2A ± Lamivudine: Results of 5-year post-treatment follow up. J Hepatol 2009;50:S336.
- Lampertico P, Vigano M, Di Costanzo G, et al. 98 extended (2 years) treatment with peginterferon alfa-2A [40KD] improves sustained response rates in genotype d patients with hbeag negative chronic hepatitis B. J Hepatol 52:S45. [Crossref]
- Lampertico P, Viganò M, Colombo M. Treatment of HBeAg-negative chronic hepatitis B with pegylated interferon. Liver Int 2011;31 Suppl 1:90-4. [Crossref] [PubMed]
- Kaymakoglu S, Oguz D, Gur G, et al. Pegylated interferon Alfa-2b monotherapy and pegylated interferon Alfa-2b plus lamivudine combination therapy for patients with hepatitis B virus E antigen-negative chronic hepatitis B. Antimicrob Agents Chemother 2007;51:3020-2. [Crossref] [PubMed]
- Piccolo P, Lenci I, Demelia L, et al. A randomized controlled trial of pegylated interferon-alpha2a plus adefovir dipivoxil for hepatitis B e antigen-negative chronic hepatitis B. Antivir Ther 2009;14:1165-74. [Crossref] [PubMed]
- Bourliere M, Rabiega P, Ganne-Carrie N, et al. HBsAg clearance after addition of 48 weeks of pegifn in hbeag negative CHB patients on nucleos(T)IDE therapy with undetectable HBVDNA for at least one year: Final results from ANRS-HB06 pegan study: Multicenter randomized controlled phase III trial. J Hepatol 2015;62:S249. [Crossref]


