Endoscopic submucosal dissection for duodenal tumors
Introduction
Endoscopic submucosal dissection (ESD) is widely adopted as an effective treatment strategy for esophageal, gastric, and colonic neoplasms. Recently, the therapeutic indication has been expanded to the duodenal neoplasms in many institutions, but it causes a lot of controversy because of unknown clinicopathological characteristics of the tumors and high incidence rate of complications associated with endoscopic procedures.
Clinicopathological characteristics of duodenal tumors
The prevalence of duodenal epithelial neoplasms is reported to be 0.03% to 0.4% of the patients undergoing the upper endoscopy (1,2), which is smaller than those of other digestive neoplasms. Majority of them are diagnosed as adenomas or mucosal adenocarcinomas located at descending part of the duodenum (3-6). The malignant transformation rate of adenomas differs depending on the previous reports. Some suggested the duodenal adenomas progressed to adenocarcinomas in 30% to 80% of the cases (7,8), but Okada reported that only 4.7% of 47 duodenal adenomas progressed to the adenocarcinoma during follow-up and pointed out a high risk of progression to adenocarcinoma for high grade adenomas of 20 mm or more in size (9). The incidence rate of lymph node metastasis of duodenal adenocarcinomas is also unclear because of the lack of cases treated by surgical resection. Some previous case-series reports showed the mucosal cancers were free from lymph node metastasis, suggesting the endoscopic therapeutic indication for them (10,11).
Merits and faults of ESD and EMR
There are basically two ways of endoscopic resection of duodenal tumors, ESD and endoscopic mucosal resection (EMR). The advantage of ESD to EMR is higher en bloc resection rate and lower local recurrence rate regardless of the tumor size, location, and submucosal fibrosis, which has been much proven in the endoscopic treatment for esophageal, gastric, and colonic tumors (12). However, those advantages may be small for the endoscopic treatment for duodenal tumors compared with that for other digestive tumors (Tables 1,2). EMR seems inferior to ESD in en bloc resection and local recurrence without restriction of duodenal tumor size. However, several reports suggested a similarly good prognosis of the cases after piecemeal resection as those after en bloc resection (13-16), and the incidence of complication was apparently more frequent in ESD than in EMR, especially for the duodenal perforation (Tables 1,2). Intraoperative perforation and delayed perforation after ESD were reported to be 6.3–50% and 0–14.3%, respectively (3-6). Recently, Ono et al. analyzed reported the clinical short-term outcomes of 1397 patients, who were endoscopicaly treated for duodenal neoplasms, by using questionnaire data taken from thirteen advanced institutions in Japan (4). In this large number of analysis, intraoperative perforation and delayed perforation each occurred in 12.1% (54/445) and 4.0% (18/445) of the cases of ESD and 1.6% (13/798) and 0.6% (5/798) of the cases of EMR, showing a higher incidence of perforation in the cases of ESD. Additionally, emergency surgery was performed in 5.4% (24/445) of the cases of ESD and 0.4% (3/798) of the cases of EMR, and it was more frequently required in the cases of delayed perforation (52.2%: 12/23) than in the cases of intraoperative perforation (19.4%: 13/67). These results of advanced institutions in Japan show the difficulty to decrease the incidence of duodenal perforation associated with ESD only by the technical improvement.
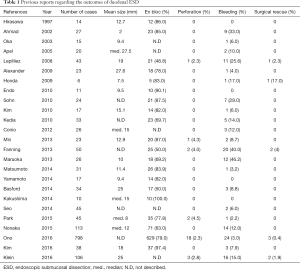
Full table
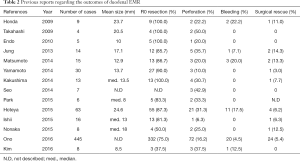
Full table
The clinical characteristics of the perforation are different between ESD and EMR. ESD-associated perforation usually occurs as a small linear hole at early stage and tears easily to be gradually enlarged along with the endoscopic procedures. Additionally, the endoscopic resection becomes technically more difficult to continue (Figure 1). On the other hand, EMR-associated perforation occurs as a small roundish hole by an incidental whole layer resection on snaring. However, the lesion has already been resected, and it is usually relatively easy to close the small perforation by using clipping devices.
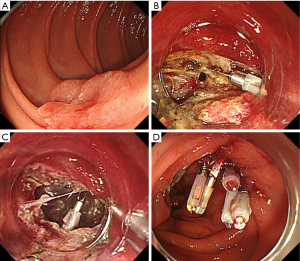
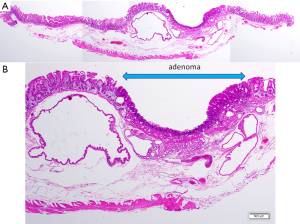
Some specific anatomical features of the duodenum can be given as the reason why the duodenal ESD frequently complicates a perforation. First, the narrow, crooked, and deeply located lumen makes it difficult to keep an adequate visual field for ESD. Second, the submucosal injection is difficult due to abundant Brunner’s glands and fibrosis in the submucosal layer, leading to the intraoperative severe conditions for submucosal dissection. Lastly, the duodenal muscle layer is very thin compared with other digestive tract (Figure 2), which is probably the biggest cause of perforation by minor physical or chemical damage including burning effect, compression of endoscopic devices, and tissue damage by bile and pancreatic juice.
Countermeasure for the duodenal perforations by ESD and EMR
Some countermeasures for the duodenal perforation have been suggested; carbon dioxide (CO2) supply, prophylactic clipping, polyglycolic acid (PGA) sheets shielding, and the laparoscopic and endoscopic cooperative surgery (LECS). Using CO2 for the air supply during ESD is necessary for the possible intraoperative perforation, regardless of the kind of digestive tract (12). The following two measures aim not for the prevention of an intraoperative perforation but for that of a delayed perforation. Prophylactic mucosal closure by clipping device after ESD is effective for the protection of the exposed muscle layer (Figure 3). However, the achievement depends on the size and location of the resected bed and scope instability (16,17). PGA sheet, an absorbable mesh used to seal tissue defects with fibrin glue, was reported to be effective for the prevention of delayed bleeding after gastric ESD (18) and postoperative stenosis after esophageal extensive ESD (19). Recently, this shielding method has been reported to be also effective for the prevention of delayed perforation after duodenal ESD (20). LECS has been developed as a minimally invasive surgery for the gastrointestinal stromal tumor (21), and also proposed as an alternative method of ESD for the case of early stage digestive cancers which is difficult to treat by ESD. Some case series reports have suggested the efficacy of LECS for the treatment of duodenal tumors; the partial duodenal resection or seromuscular suturing of resection bed after ESD by using LECS technique (22,23). However, the extensive resection by LECS is considered to cause the postoperative stenosis, and is inadaptable to the lesions near the duodenal papilla.

Current status and future prospects of duodenal ESD
Thus, duodenal ESD has not yet reached the recommendable measure for the endoscopic treatment of duodenal tumors. To overwhelm the various problems concerning the duodenal ESD, the endoscopic skill-up is necessary but insufficient, because a high incidence of complications has been experienced in the advanced Japanese institutions. Nevertheless, to reduce the incidence of complications as much as possible, the cases of possible indication of duodenal ESD should be collected to such specific institutions with advanced techniques and experiences. Additionally, the lesions likely to be resectable by EMR should not be treated by ESD, and we should have the prudence in aggressively performing duodenal ESD.
The complication of duodenal ESD we should overcome the most is an intraoperative perforation, which is most frequently experienced but has a lack of effective preventive measures. It seems difficult to prevent the intraoperative perforation singly by the progress of ESD techniques or related devices, implying the necessity of a fundamental change of the therapeutic method. From the perspective of a combined treatment, the further radical progress of LECS may possibly change the treatment for the duodenal tumors to more safe and feasible one.
Conclusions
The duodenal ESD still have many unsolved problems regarding feasibility and safety even by introducing various ideas, and the indication should be well discussed in consideration of the expected therapeutic effect and complications.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jepsen JM, Persson M, Jakobsen NO, et al. Prospective study of prevalence and endoscopic and histopathologic characteristics of duodenal polyps in patients submitted to upper endoscopy. Scand J Gastroenterol 1994;29:483-7. [Crossref] [PubMed]
- Jung SH, Chung WC, Kim EJ, et al. Evaluation of non-ampullary duodenal polyps: comparison of non-neoplastic and neoplastic lesions. World J Gastroenterol 2010;16:5474-80. [Crossref] [PubMed]
- Fujihara S, Mori H, Kobara H, et al. Management of a large mucosal defect after duodenal endoscopic resection. World J Gastroenterol 2016;22:6595-609. [Crossref] [PubMed]
- Ono H, Kaise M, Nonaka S, et al. Clinical Issues of Duodenal Endoscopic Treatment. Stomach and Intestine 2016; 51: 1585-92. Available online: http://medicalfinder.jp/doi/10.11477/mf.1403200770
- Kim TW, Kim GH, Park DY, et al. Endoscopic resection for duodenal subepithelial tumors: a single-center experience. Surg Endosc. 2017;31:1936-46. [PubMed]
- Klein A, Nayyar D, Bahin FF, et al. Endoscopic mucosal resection of large and giant lateral spreading lesions of the duodenum: success, adverse events, and long-term outcomes. Gastrointest Endosc 2016;84:688-96. [Crossref] [PubMed]
- Sakorafas GH, Friess H, Dervenis CG. Villous tumors of the duodenum: biologic characters and clinical implications. Scand J Gastroenterol 2000;35:337-44. [Crossref] [PubMed]
- Galandiuk S, Hermann RE, Jagelman DG, et al. Villous tumors of the duodenum. Ann Surg 1988;207:234-9. [Crossref] [PubMed]
- Okada K, Fujisaki J, Kasuga A, et al. Sporadic nonampullary duodenal adenoma in the natural history of duodenal cancer: a study of follow-up surveillance. Am J Gastroenterol 2011;106:357-64. [Crossref] [PubMed]
- Abbass R, Rigaux J, Al-Kawas FH. Nonampullary duodenal polyps: characteristics and endoscopic management. Gastrointest Endosc 2010;71:754-9. [Crossref] [PubMed]
- Poultsides GA, Huang LC, Cameron JL, et al. Duodenal adenocarcinoma: clinicopathologic analysis and implications for treatment. Ann Surg Oncol 2012;19:1928-35. [Crossref] [PubMed]
- Technology status report evaluation. Endoscopic mucosal resection. Gastrointest Endosc 2000;52:860-3. [Crossref] [PubMed]
- Alexander S, Bourke MJ, Williams SJ, et al. EMR of large, sessile, sporadic nonampullary duodenal adenomas: technical aspects and long-term outcome (with videos). Gastrointest Endosc 2009;69:66-73. [Crossref] [PubMed]
- Fanning SB, Bourke MJ, Williams SJ, et al. Giant laterally spreading tumors of the duodenum: endoscopic resection outcomes, limitations, and caveats. Gastrointest Endosc 2012;75:805-12. [Crossref] [PubMed]
- Maruoka D, Arai M, Kishimoto T, et al. Clinical outcomes of endoscopic resection for nonampullary duodenal high-grade dysplasia and intramucosal carcinoma. Endoscopy 2013;45:138-41. [Crossref] [PubMed]
- Nonaka S, Oda I, Tada K, et al. Clinical outcome of endoscopic resection for nonampullary duodenal tumors. Endoscopy 2015;47:129-35. [PubMed]
- Hoteya S, Kaise M, Iizuka T, et al. Delayed bleeding after endoscopic submucosal dissection for non-ampullary superficial duodenal neoplasias might be prevented by prophylactic endoscopic closure: analysis of risk factors. Dig Endosc 2015;27:323-30. [Crossref] [PubMed]
- Tsuji Y, Fujishiro M, Kodashima S, et al. Polyglycolic acid sheets and fibrin glue decrease the risk of bleeding after endoscopic submucosal dissection of gastric neoplasms (with video). Gastrointest Endosc 2015;81:906-12. [Crossref] [PubMed]
- Iizuka T, Kikuchi D, Yamada A, et al. Polyglycolic acid sheet application to prevent esophageal stricture after endoscopic submucosal dissection for esophageal squamous cell carcinoma. Endoscopy 2015;47:341-4. [PubMed]
- Takimoto K, Imai Y, Matsuyama K. Endoscopic tissue shielding method with polyglycolic acid sheets and fibrin glue to prevent delayed perforation after duodenal endoscopic submucosal dissection. Dig Endosc 2014;26 Suppl 2:46-9. [Crossref] [PubMed]
- Hiki N, Yamamoto Y, Fukunaga T, et al. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc 2008;22:1729-35. [Crossref] [PubMed]
- Tamaki I, Obama K, Matsuo K, et al. A case of primary adenocarcinoma of the third portion of the duodenum resected by laparoscopic and endoscopic cooperating surgery. Int J Surg Case Rep 2015;9:34-8. [Crossref] [PubMed]
- Irino T, Nunobe S, Hiki N, et al. Laparoscopic-endoscopic cooperative surgery for duodenal tumors: a unique procedure that helps ensure the safety of endoscopic submucosal dissection. Endoscopy 2015;47:349-51. [PubMed]


