Sternal wound management after bilateral internal thoracic artery grafting: a significant detail
Introduction
Throughout the last 20 years, many—prospective and retrospective—observational studies have shown long-term survival benefits derived from the use of bilateral internal thoracic artery (BITA) grafts for myocardial revascularization (BITA grafting). Outcomes of large cohorts of BITA patients have been reviewed. Pooled analyses of these studies suggest that, at 10 years, there are approximately 20% fewer all-cause deaths with BITA grafting than with the standard model of myocardial revascularization, i.e., single internal thoracic artery (SITA) graft to the left anterior descending coronary artery (SITA grafting), and saphenous vein grafts (SVGs) for the remaining diseased coronary vessels (1-3). The benefits arise mainly from the higher late patency of internal thoracic artery (ITA) graft versus SVG, even for difficult subset of patients such as diabetics. In fact, the SITA graft has a 10-year rate of angiographic patency exceeding 90%, as compared with 50% for SVG, having the radial artery (RA) an intermediate performance (4-6). However, BITA grafts remain underutilized in coronary surgery, and routine use of BITA grafting is still far from coming in most worldwide institutions (7,8). Increased risk of postoperative complications, primarily sternal wound infection (SWI) and bleeding, longer duration of operation due to the time needing for the second ITA graft harvesting, as well as more complex surgical techniques limit a more extensive adoption of surgical strategies that require the simultaneous use of both ITAs. In lack of randomized evidence of long-term benefits, these issues are valid deterrents to discourage liberal BITA use (1-3,7-10).
Results at 5 years of The Arterial Revascularization Trial (ART)
On the basis of the above considerations, the interim analysis of clinical and safety outcomes at 5 years of ART that has been recently reported by Taggart and colleagues is welcome (11). Undoubtedly, these authors should be congratulated for their excellent and informative study.
This two-group, multicenter, randomized trial was initiated in 2004 and conducted in 28 cardiac surgical centers in seven countries. Its primary objective was to compare 10-year survival rates associated with BITA and SITA grafting. Secondary outcomes were the composite of death from any cause, myocardial infarction, or stroke, rate of repeat revascularization, safety outcomes (including bleeding and sternal wound complications), quality of life, costs, and cost effectiveness. Data were gathered at participating sites by means of annual telephone calls or hospital visits. Serious adverse events were reported by investigators on specific forms. Quality of life was assessed with the use of ad hoc well-codified questionnaires. The trial was sponsored by the University of Oxford. Trial management was provided initially by the Clinical Trials and Evaluation Unit at the Royal Brompton and Harefield NHS Foundation Trust in London and from 2014 by the Surgical Intervention Trials Unit at the University of Oxford (11-13).
Eligible patients were those with multivessel coronary disease who were scheduled to undergo coronary surgery (including patients requiring urgent surgery but not those with evolving myocardial infarction). Patients requiring only single grafts or concomitant valve surgery, as well as those with a history of coronary surgery, were excluded. Patients were randomly assigned, in a 1:1 ratio, to undergo SITA or BITA grafting.
The group that underwent SITA grafting received a SITA graft to the left anterior descending coronary artery plus supplemental saphenous vein or RA grafts to other coronary arteries. The group that underwent BITA grafting received both left and right ITA grafts to the two most important coronary arteries on the left side with supplemental saphenous vein or RA grafts to other coronary arteries (left-sided BITA grafting). Anastomosis of an ITA graft to the right coronary artery was not permitted because of concerns about inferior long-term patency. Surgeons could participate in the trial only if their experience included 50 or more operations using BITA grafts. Standard methods for anesthesia and myocardial protection were used according to local practice.
A total of 3,102 patients operated on between June 2004 and December 2007 were enrolled into the study; 1,554 patients were randomly assigned to the SITA group and 1,548 to the BITA group. The groups were well matched with respect to age, sex, race and ethnic origin, body mass index, systolic and diastolic blood pressure, smoking status, and coexisting conditions. In the SITA group, 96.1% of the patients received a SITA graft, and in the BITA group, 83.6% of the patients received BITA grafts. The rate of non-adherence to BITA graft surgery was higher than expected. Off-pump procedures were performed in 40.6% of patients. The mean number of grafts in each group was three. Medications at 5 years were well balanced between the two groups.
A total of 159 (5.1%) participants had unknown vital status at 5 years because of loss to follow-up or withdrawal from the trial. At 5 years of follow-up, there were 134 deaths (8.7%) in the BITA group and 130 deaths (8.4%) in the SITA group [hazard ratio (HR) with the SITA group as the control group throughout, 1.04; 95% confidence interval (CI), 0.81–1.32; P=0.77]. Results were similar after adjustment for age, sex, diabetes status, and ejection fraction (HR, 1.03; 95% CI, 0.81–1.32; P=0.80). For the composite of death from any cause, myocardial infarction, or stroke, there were 189 (12.2%) in the BITA group and 198 events (12.7%) in the SITA group (HR, 0.96; 95% CI, 0.79–1.17; P=0.69). Approximately half the deaths were classified as being cardiovascular, with a HR that was similar to that in the analysis of all-cause mortality. Subgroup analyses did not show any evidence of significant interactions.
The incidence of sternal wound reconstruction was 1.9% in the BITA group, as compared with 0.6% in the SITA group (relative risk, 2.91; 95% CI, 1.42–5.95; P=0.002), and all these events occurred in the first year after surgery. Sternal wound complications occurred in approximately twice as many patients in the BITA group as in the SITA group, whereas the rates of major bleeding events and the need for any repeat revascularization were similar in the two groups (just over 6% in each group). Angina status at 5 years showed similar results in the two groups, with approximately 70% of the patients who responded to the questionnaire reporting no chest pain. Mean quality-of-life scores at 5 years showed no between group differences for patients who provided data.
In a nutshell, in the ART, patients undergoing coronary surgery were randomly assigned to receive either SITA or BITA grafting. At 5 years of follow-up, there were no significant differences in clinical outcomes between the two groups. There was some early excess of sternal wound complications in the BITA group. Ten year follow-up is ongoing.
The absence of any midterm benefit from BITA over SITA grafting might have several explanations.
The rate of SVG failure within 5 years may not be high enough to have an obvious adverse clinical effect. There may not be a direct association between SVG failure and clinical events. Variation in surgeon’s experience may have reduced the effectiveness of BITA grafting. There may be little difference between the effects of the two techniques on clinical outcomes, owing to better long-term SVG patency, asymptomatic SVG failure, and improved medical therapy.
According to Taggart and colleagues, the following limitations of the trial have to be considered: (I) this planned interim analysis of an ongoing trial does not provide definitive long-term evidence regarding the comparison between SITA and BITA grafting (which is still awaited); (II) at 5 years, the trial has less power to detect a difference in outcomes than is likely to be the case at 10 years, with consequent wide CI for the primary outcome; (III) more patients who were randomly assigned to BITA than to SITA grafting did not receive the assigned procedure (16.4% vs. 3.9%), and some expected loss to follow-up may reduce the power of the trial.
Comment
While I totally agree with the critical analysis by the authors, I have some comments in addition.
Generally, the standard model of myocardial revascularization does not provide the use of RA, but of SVGs alone in addition to the SITA graft to the left anterior descending coronary artery. In the ART, instead, some SITA patients have received a RA graft as well. Consequently, for these patients, the comparison was between surgery using two arterial grafts, ITA plus RA, and surgery using two arterial grafts, right ITA plus left ITA! (14-16).
In the ART, surgeons could adopt a variety of configurations for BITA grafting. Generally, BITA grafts are used in two configurations: (I) both ITAs retain their connection to the corresponding subclavian artery (BITA in situ, or double source configuration); (II) the right ITA is taken down and used as a free graft from the in situ left ITA (BITA Y-graft, or single source configuration). Although to date there is no evidence of superiority of the one configuration over the other (and the choice either of the one or the other configuration depends on the site of target coronary vessels, the length of BITA conduits, and ultimately the surgeon’s preference), this aspect could have any impact on efficacy of revascularization.
There was a mean number of three coronary grafts per patient. It is not clear for me whether this number was the mean number of coronary anastomoses per patient as well. If yes, there could be some issues about the completeness of revascularization. If not, how many sequential anastomoses were performed?
When the analysis of the results at 10 years will be performed, these three points should be taken into account.
Indications to the use of off-pump surgery, important issues such as the use of the “skeletonized”, “semi-skeletonized”, or “pedicled” technique for ITA harvesting, and the relationship between these two aspects and the rate of sternal wound complications after surgery were addressed partially, in post hoc nonrandomized analyses (16-18). Actually, although pre-specified subgroup analyses were performed on the basis of surgery type, RA grafting, and number of grafts, I believe that the above reported specific issues remain unresolved.
Last but not least, insufficient data are recorded as the perioperative management of sternal wounds and the postoperative management of sternal wound complications.
In conclusion, I think that the use of BITA grafting is really a more complex technique that requires for surgeons specific skills and sufficient expertise, which are hardly obtained after only 50 procedures. Besides, a multidisciplinary, preoperative, intraoperative, and postoperative approach is needed to prevent and manage successfully sternal wound complications. Throughout the years, each centre should develop a specific program to control sternal complications after sternotomy. The program should be based both on the experience and expertise of the surgeons and the evidence of the literature (19). A definite program of prevention and treatment of SWIs after BITA grafting has been created and adopted at the Cardiovascular Department of the University Hospital of Trieste, Italy (Table 1) (9,20,21).
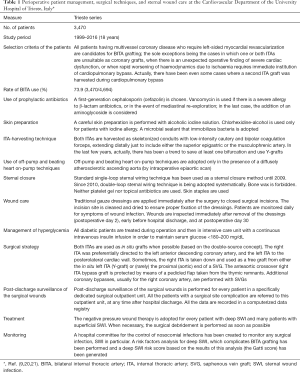
Full table
I think that Dr. David P. Taggart, his colleagues, and the ART Investigators should be congratulated for their excellent study.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Taggart DP, D’Amico R, Altman DG. Effect of arterial revascularisation on survival: a systematic review of studies comparing bilateral and single internal mammary arteries. Lancet 2001;358:870-5. [Crossref] [PubMed]
- Takagi H, Goto SN, Watanabe T, et al. A meta-analysis of adjusted hazard ratios from 20 observational studies of bilateral versus single internal thoracic artery coronary artery bypass grafting. J Thorac Cardiovasc Surg 2014;148:1282-90. [Crossref] [PubMed]
- Yi G, Shine B, Rehman SM, et al. Effect of bilateral internal mammary artery grafts on long-term survival: a meta-analysis approach. Circulation 2014;130:539-45. [Crossref] [PubMed]
- Fitzgibbon GM, Kafka HP, Leach AJ, et al. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol 1996;28:616-26. [Crossref] [PubMed]
- Gansera B, Schmidtler F, Angelis I, et al. Patency of internal thoracic artery compared to vein grafts - postoperative angiographic findings in 1189 symptomatic patients in 12 years. Thorac Cardiovasc Surg 2007;55:412-7. [Crossref] [PubMed]
- Calafiore AM, Contini M, Vitolla G, et al. Bilateral internal thoracic artery grafting: long-term clinical and angiographic results of in situ versus Y grafts. J Thorac Cardiovasc Surg 2000;120:990-6. [Crossref] [PubMed]
- Tabata M, Grab JD, Khalpey Z, et al. Prevalence and variability of internal mammary artery graft use in contemporary multivessel coronary artery bypass graft surgery: analysis of the Society of Thoracic Surgeons National Cardiac Database. Circulation 2009;120:935-40. [Crossref] [PubMed]
- Tatoulis J, Buxton BF, Fuller JA. The right internal thoracic artery: the forgotten conduit — 5,766 patients and 991 angiograms. Ann Thorac Surg 2011;92:9-15. [Crossref] [PubMed]
- Gatti G, Dell'Angela L, Barbati G, et al. A predictive scoring system for deep sternal wound infection after bilateral internal thoracic artery grafting. Eur J Cardiothorac Surg 2016;49:910-7. [Crossref] [PubMed]
- Gatti G, Perrotti A, Reichart D, et al. Glycated Hemoglobin and Risk of Sternal Wound Infection After Isolated Coronary Surgery. Circ J 2016;81:36-43. [Crossref] [PubMed]
- Taggart DP, Altman DG, Gray AM, et al. Randomized Trial of Bilateral versus Single Internal-Thoracic-Artery Grafts. N Engl J Med 2016;375:2540-9. [Crossref] [PubMed]
- Taggart DP, Lees B, Gray A, et al. Protocol for the Arterial Revascularisation Trial (ART). A randomised trial to compare survival following bilateral versus single internal mammary grafting in coronary revascularisation Trials 2006;7:7. [ISRCTN46552265]. [Crossref] [PubMed]
- Taggart DP, Altman DG, Gray AM, et al. Randomized trial to compare bilateral vs. single internal mammary coronary artery bypass grafting: 1-year results of the Arterial Revascularisation Trial (ART). Eur Heart J 2010;31:2470-81. [Crossref] [PubMed]
- Collins P, Webb CM, Chong CF, et al. Radial artery versus saphenous vein patency randomized trial: five-year angiographic follow-up. Circulation 2008;117:2859-64. [Crossref] [PubMed]
- Tatoulis J, Buxton BF, Fuller JA, et al. Long-term patency of 1108 radial arterial coronary angiograms over 10 years. Ann Thorac Surg 2009;88:23-9. [Crossref] [PubMed]
- Benedetto U, Raja SG, Albanese A, et al. Searching for the second best graft for coronary artery bypass surgery: a network meta-analysis of randomized controlled trials†. Eur J Cardiothorac Surg 2015;47:59-65. [Crossref] [PubMed]
- Benedetto U, Altman DG, Gerry S, et al. Pedicled and skeletonized single and bilateral internal thoracic artery grafts and the incidence of sternal wound complications: insights from the Arterial Revascularization Trial. J Thorac Cardiovasc Surg 2016;152:270-6. [Crossref] [PubMed]
- Taggart DP, Altman DG, Gray AM, et al. Effects of on-pump and off-pump surgery in the Arterial Revascularization Trial. Eur J Cardiothorac Surg 2015;47:1059-65. [Crossref] [PubMed]
- Kieser TM, Rose MS, Aluthman U, et al. Toward zero: deep sternal wound infection after 1001 consecutive coronary artery bypass procedures using arterial grafts: implications for diabetic patients. J Thorac Cardiovasc Surg 2014;148:1887-95. [Crossref] [PubMed]
- Gatti G, Soso P, Dell'Angela L, et al. Routine use of bilateral internal thoracic artery grafts for left-sided myocardial revascularization in insulin-dependent diabetic patients: early and long-term outcomes. Eur J Cardiothorac Surg 2015;48:115-20. [Crossref] [PubMed]
- Gatti G, Pappalardo A, Gon L, et al. Protecting the crossover right internal thoracic artery bypass graft with a pedicled thymus flap. Ann Thorac Surg 2006;82:1919-21. [Crossref] [PubMed]


