Inhibition of SOX9 may be an effective target for increasing radiosensitivity in gastrointestinal cancer
Radiotherapy (RT) plays an important role in the treatment of gastrointestinal (GI) cancer. When combined with other anti-cancer therapy modalities, RT can increase cytotoxicity and improve treatment efficacy. However, some patients develop radioresistance and show treatment failure. In addition, certain patients experience acute enteritis during abdominal RT for GI cancer or sequelae of chronic radiation enteritis after RT. Therefore, it is important to determine the mechanism of radioresistance as well as RT-induced enteritis. Previous studies have demonstrated that two intestinal stem cells respond differentially to RT; one actively proliferating stem cell (aISC) is more radiosensitive, whereas the other reserve intestinal stem cell (rISC) is relatively radioresistant (1). Furthermore, the transcription factor Sox9 has been reported to regulate proliferation and participate in the biological function of stemness in small intestinal cells (2,3). A study published in Gastroenterology by Roche et al. showed that SOX9 is required for the production and maintenance of label-retaining cells (4). In their study, Roche et al. showed that SOX9 knockout intestinal epithelium lacked regeneration capacity after RT, whereas SOX9 knockout intestinal crypts underwent apoptosis after RT irrespective of cell cycle arrest and DNA repair (4). These findings indicate that SOX9 plays key roles in mediating stemness and radioresistance in intestinal stem cells (ISCs). Thus, it may be possible to improve efficiency and develop potential strategies involving RT for GI cancer by controlling the expression of SOX9 in stem cells.
Several studies suggested that a small subpopulation of malignant cells, known as cancer stem-like cells (CSCs) or tumor-initiating cells, possesses properties of stem cell such as self-renewal and differentiation; the self-renewal property is thought to be the origin of tumor growth and development (5). In addition, CSCs exhibits resistance to current anti-cancer therapeutics, leading to tumor metastasis and recurrence after treatment (6). Therefore, targeting of CSCs may be useful in cancer therapeutics. The crucial role played by SOX9 in CSCs has been verified in digestive system cancers, including pancreatic cancer and hepatocellular carcinoma (7-9). In a study of pancreatic cancer, Sun et al. found that the nuclear factor-κB signaling pathway can epigenetically regulate the expression of SOX9 and promote the invasiveness of CSCs (7). Grimont et al. also demonstrated that SOX9 upregulated the ERBB signaling pathway to promote the tumorigenesis of pancreatic cancer (8). In addition, SOX9 is necessary for tumor cell initiation and division, self-renewal, and tumorigenicity in CSCs of hepatocellular carcinoma (9). In a xenograft mouse model of lung cancer, Luanpitpong et al. showed that SOX9 stabilization regulated by SLUG is important for the expansion and metastasis of CSCs (10). Taken together, continuously increased expression of SOX9 may be associated with the maintenance of certain phenotypes of CSCs and contribute to the propagation of several tumor types. On the other hand, SOX9 is a novel cancer stem cell marker and inhibition of SOX9 may be an effective target for eradicating CSCs.
However, the exact role of SOX9 in the mechanism of resistance to cancer therapy remains unclear, particularly for RT. Based on the relationships among SOX9, CSCs, and resistance, a novel therapeutic strategy related to radioresistance should be developed to eradicate GI cancer. A previous report showed that drug-loaded nanocarriers conjugated with ligands targeted to CSC possess higher target selectivity and effectively release drugs within CSCs (11). Currently, glycans bound to proteins on cell membrane are regarded as potentially specific CSC markers, which differ from normal stem cells. If SOX9 inhibitors or siRNA can be transported by this specific nanocarrier, we can investigate whether nanocarrier-conjugated SOX9 inhibitors can enhance the RT therapeutic effects in GI cancer, such as pancreatic cancer, hepatocellular carcinoma, and colorectal cancer, using in vitro cell lines and in vivo xenograft or orthotopic graft models. These approaches will reveal the precise mechanisms of radioresistance involving SOX9 and its regulated signaling in CSCs. In addition to enhanced RT sensitivity, ablation of SOX9 inhibits the extension of CSCs and lessens the invasiveness and metastases of cancer cells. A therapeutic strategy combining SOX9 inhibition and RT should be developed for GI cancer.
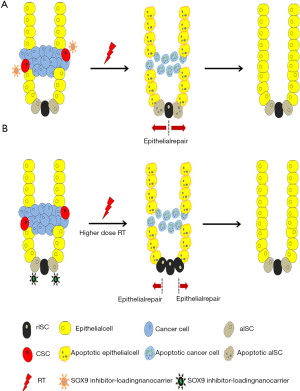
Notably, the use of SOX9 inhibitors combined with RT may cause adverse effects, such as enteritis, by reducing aISC-associated intestinal epithelium regeneration. Previous studies demonstrated that RelA, a transcription factor that plays a crucial role in biological processes, and Fgf8 can induce SOX9 expression (12,13). If SOX9 or its inducers can be delivered to normal intestinal tissue specifically and expressed successfully, rISC function can be significantly upregulated to protect against high-dose RT-associated enteritis. If the hypothesis is confirmed, this therapeutic strategy may effectively attenuate RT-induced GI injury and further promote crypt repair and regeneration after the completion of RT. These findings suggest that the importance of determining the applications of diverse clinical therapeutics of SOX9 in GI cancers, such as the use of SOX9 inhibitors in concurrent RT and use of SOX9 inducers after completion of high-dose RT (Figure 1).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol 2014;15:19-33. [Crossref] [PubMed]
- Formeister EJ, Sionas AL, Lorance DK, et al. Distinct SOX9 levels differentially mark stem/progenitor populations and enteroendocrine cells of the small intestine epithelium. Am J Physiol Gastrointest Liver Physiol 2009;296:G1108-18. [Crossref] [PubMed]
- Mori Akiyama Y, van den Born M, van Es JH, et al. SOX9 is required for the differentiation of paneth cells in the intestinal epithelium. Gastroenterology 2007;133:539-46. [Crossref] [PubMed]
- Roche KC, Gracz AD, Liu XF, et al. SOX9 maintains reserve stem cells and preserves radioresistance in mouse small intestine. Gastroenterology 2015;149:1553-63.e10. [Crossref] [PubMed]
- Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med 2006;355:1253-61. [Crossref] [PubMed]
- Boman BM, Huang E. Human colon cancer stem cells: a new paradigm in gastrointestinal oncology. J Clin Oncol 2008;26:2828-38. [Crossref] [PubMed]
- Sun L, Mathews LA, Cabarcas SM, et al. Epigenetic regulation of SOX9 by the NF-κB signaling pathway in pancreatic cancer stem cells. Stem Cells 2013;31:1454-66. [Crossref] [PubMed]
- Grimont A, Pinho AV, Cowley MJ, et al. SOX9 regulates ERBB signalling in pancreatic cancer development. Gut 2015;64:1790-9. [Crossref] [PubMed]
- Liu C, Liu L, Chen X, et al. Sox9 regulates self-renewal and tumorigenicity by promoting symmetrical cell division of cancer stem cells in hepatocellular carcinoma. Hepatology 2016;64:117-29. [Crossref] [PubMed]
- Luanpitpong S, Li J, Manke A, et al. SLUG is required for SOX9 stabilization and functions to promote cancer stem cells and metastasis in human lung carcinoma. Oncogene 2016;35:2824-33. [Crossref] [PubMed]
- Lu B, Huang X, Mo J, et al. Drug Delivery Using Nanoparticles for Cancer Stem-Like Cell Targeting. Front Pharmacol 2016;7:84. [Crossref] [PubMed]
- Monsoro-Burq AH, Fletcher RB, Harland RM. Neural crest induction by paraxial mesoderm in Xenopus embryos requires FGF signals. Development. 2003;130:3111-24. [Crossref] [PubMed]
- Ushita M, Saito T, Ikeda T, et al. Transcriptional induction of SOX9 by NF-kappaB family member RelA in chondrogenic cells. Osteoarthritis Cartilage 2009;17:1065-75. [Crossref] [PubMed]


