Animal models of hospital-acquired pneumonia: current practices and future perspectives
Hospital-acquired pneumonia
Epidemiology of hospital-acquired pneumonia
Hospital-acquired pneumonia (HAP) is the second most common nosocomial infection (1), and is characterized by high morbidity and mortality (2). HAP is frequently caused by either multidrug-resistant nosocomial bacteria or by opportunistic pathogens, i.e., microorganisms that usually do not cause an infection in healthy individuals but can typically colonize and infect critically ill patients. HAP is especially a serious threat to patients hospitalized in the intensive care unit (ICU) and receiving mechanical ventilation. This so called ventilator-associated pneumonia (VAP) is defined as a pneumonia that typically develops more than 48 hours after endotracheal intubation and initiation of mechanical ventilation (3,4). Mechanical ventilation significantly increases the risk for infections resulting in a 20-fold increased risk for developing pneumonia as compared to non-ventilated patients in the ICU (5,6). VAP is the most common nosocomial infection in ICU settings (7,8), and after controlling for other variables, patients developing VAP have a considerably higher mortality, reaching up to 50% in some studies (9,10), compared to non-VAP pneumonia patients (11,12).
Etiology of hospital acquired pneumonia
HAP is mostly caused by opportunistic pathogens such as Pseudomonas aeruginosa, methicillin-resistant Staphylococcus aureus (MRSA), Acinetobacter baumannii and Enterobacteriaceae that tend to colonize patients very quickly once admitted to the hospital (13,14). In particular, infections caused by Gram-negative multidrug-resistant organisms, including P. aeruginosa and extended-spectrum β-lactamase-producing or carbapenemase-producing Enterobacteriaceae, are increasingly being reported worldwide (15). Especially in VAP, P. aeruginosa is one of the main etiologic agents responsible for a global prevalence rate of >25% (16) and is associated with development of other serious complications such as septic shock and multiple organ dysfunction (17).
For this review, we consider animal cystic fibrosis (CF) models as highly relevant to understand the pathophysiology of HAP because of their shared etiology. P. aeruginosa is, as in VAP, a major cause of pulmonary infection in CF patients (18), along with other pathogens known for their biofilm producing capacity such as Staphylococcus aureus and Burkholderia cepacia (19). Cystic fibrosis is the most common and fatal autosomal-recessive disease in the Caucasian population affecting ≈70,000 individuals worldwide (20) and is caused by a dysfunctional CF transmembrane conductance regulator (CFTR) (21,22) resulting in increased mucous secretion in the alveolar spaces that provide an ideal environment for bacterial colonization and biofilm formation (23). This biofilm protects bacteria from host immune cells and antibiotics by encapsulation and sequestration (24,25) and thus co-induces the typically persistent type of lung inflammation observed in CF patients (26). Moreover, VAP pathogenesis is also closely linked to biofilm forming organisms colonizing the endotracheal tube (ETT) such as P. aeruginosa, and the presence of P. aeruginosa in the biofilm on the ETT microbiome negatively correlates with patient prognosis (27).
Animal models of hospital-acquired pneumonia
Need for animal modeling
For many decades now, animal models of infection are increasingly being utilized in medical research and are responsible for accelerated progress in various fields such as cancer, neuroscience and most importantly, infectious diseases and drug development. The aim of developing an animal model to study pneumonia is to mimic the pathophysiologic and phenotypic characteristics seen in humans in a more controlled setting. Such models give a more accurate control of significant variables through the course of infection by minimizing confounders like co-morbidities or antibiotic use. Studying animal models also has other advantages including circumventing sampling limitation issues that are commonly encountered with human subjects. Precise control over timing of the infectious challenge in animal models also allows for a better understanding of temporal evolution of the disease and development of complications that are most likely related to an altered immune-inflammatory response of the host. Partial or isomorphic induced animal models of pneumonia are usually designed with the purpose of studying specific phenotypic aspects of diseases while lacking other clinical signs or etiology. Nonetheless animal models of pneumonia, and more specifically rodent pneumonia models, have aided considerably in our understanding of disease pathomechanisms and shown their utility in pre-clinical drug testing.
As discussed above, although other pathogens including Enterobacteriaceae, S. aureus and A. baumannii are important causes of HAP, P. aeruginosa is one of the most common HAP etiologies and therefore the most studied organism. Thus, in this review, the main characteristics of the most used P. aeruginosa pneumonia animal models for acute pneumonia, VAP and chronic pneumonia occurring in CF patients are summarized and compared (Table 1). Several different species have been used to model human pneumonia including piglets (28-30), rodents (31-33), primates (34,35), sheep (36,37), dogs (38,39) and rabbits (40,41) and these models have proven instructive in studies of disease mechanisms and in antibiotic testing. Nonetheless, rodents have been the preferred choice in translational pulmonary research as they not only are in accordance with the 3R principles of animal experimentation (42) but also offer specific advantages such as the potential to validate key findings or elucidate distinct pathogenic steps in a wide range of developed transgenic rodent models that are available to the scientific community.
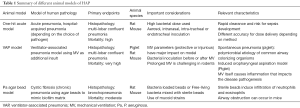
Full table
Types of pneumonia models
One hit acute pneumonia model
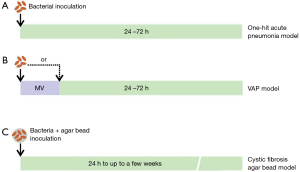
This simple model of pneumonia is established by administering a bacterial inoculum into the lungs and different methods of bacterial delivery have been described (Figure 1). Intratracheal instillation is the most used method in HAP research and involves injecting the bacterial suspension directly into the trachea or lungs followed by air for dispersion of the bolus (33,43-50). This method gives the most precise control on the delivered dose. However, surgically exposing the trachea and then suturing the incision provokes an inflammatory response in the target organ, the lung, which can have an effect on the measured endpoints. Endotracheal inoculation (Figure 2A upper panel), on the other hand, involves intubating the animal to facilitate instillation of the bacterial solution in the lungs and is as precise as the intratracheal instillation method (31,32,51). Less invasive methods include intranasal administration, whereby bacterial dose is administered in droplets through the nostrils followed by aspiration by the animal (52), or aerosol administration, that can be performed in unrestrained animals (53). However, the exact dose that reaches the lower respiratory tract in both methods is uncontrolled and animals frequently develop upper respiratory tract infections (52) or infections other than pneumonia (53). One method to control dose delivery with aerosolization is to immediately sacrifice a few (sentinel) animals after aerosolization for quantitative colony counts performed on bronchoalveolar lavage fluid or lung tissue lysates (53).
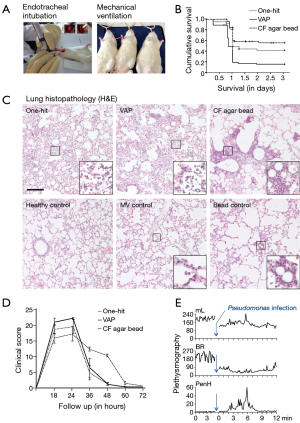
Acute P. aeruginosa pneumonia, both in rodents and humans, is characterized by a high 1- to 3-day mortality (33,50) and histologically presents as multilobar confluent pneumonia (54) causing high alveolar neutrophilic infiltration along with vascular congestion, alveolitis and alveolar collapse (Figure 2B,C). The inflammatory response towards acute P. aeruginosa pneumonia is chiefly governed by expression of proinflammatory cytokines including TNFα, IFNγ, IL-1α, IL-1β and IL-12 as well as chemotactic molecules such as IL-8 secreted by innate immune cells, epithelial cells and alveolar macrophages that result in neutrophil recruitment to the site of infection (54-57). However, the choice of inoculum dose is of high importance because a relatively high dose of P. aeruginosa causes a more marked increase in production of cytokines in the early time-points compared to lower doses (50) and thus has important consequences for studying pneumonia development.
Ventilator-associated pneumonia model
As described above, mechanical ventilation by itself is an important component in the pathogenesis of VAP, however, spontaneous development of pneumonia that occurs naturally in patients, is induced in mechanically ventilated animals most frequently by co-challenging with a bacterial inoculum. Interestingly, mechanical ventilation has been shown to cause a sterile inflammatory response in the lung leading to tissue damage caused by different mechanisms such as overstretching of the lung, barotrauma and volutrauma, leakage of air due to disruption of the airspace wall, pulmonary edema and atelectrauma (repeated opening and closing of alveoli) (58-61). Mechanical ventilation-associated lung inflammation is marked by an upregulation of proinflammatory cytokines such as TNFα, IFNγ, IL-6 and IL-1α and IL-1β (62-68) combined with chemokine release including IL-8 and CXCL-1 (69). However, mechanical ventilation also has been shown to cause a lowered natural killer cell activity and reduced MIP-2 and IL-10 expression in splenocyte proliferation assays from mechanically ventilated rats (67). Moreover, host immune response alters when different ventilation strategies are used (70) and therefore, clinically relevant ventilation strategies similar to those applied in patients of 8 mL/kg tidal volume or less should be used in animals to accurately mimic lung inflammation caused by mechanical ventilation.
To establish VAP animal models, two main strategies have been used. In the first strategy, bacteria are injected into the lungs prior to mechanical ventilation (71,72) (Figure 1). A second strategy uses bacterial instillation after ventilation and mimics the more natural disease evolution (31,32) where mechanical ventilation precedes bacterial infection occurring in VAP patients. VAP models display similar histological features as compared to the direct acute pneumonia model (Figure 2C), however, VAP animal model show an increased bacterial lung burden, a more severe disease progression and a higher mortality compared to animals that received the same bacterial dose without prior ventilation (31,32) (Figure 2B).
In piglets, it has been shown that experimentally-induced tracheal stenosis along with prolonged mechanical ventilation of up to 4 days causes spontaneous pneumonia development with endogenous microbiota as primary etiology, although known opportunistic human VAP pathogens including Pseudomonas and Klebsiella species have also been isolated in this piglet VAP model (29). Recently, a porcine P. aeruginosa VAP model was established via oropharyngeal challenge immediately after intubation and a second challenge 4 hours into ventilation using a ceftriaxone-resistant P. aeruginosa strain to ensure pulmonary aspiration of oropharyngeal secretions caused by the desired organism (28). Using 40 cm H2O pressure in the endotracheal cuff, the authors limited direct bacterial inoculation but rather mimicked aspiration of oropharyngeal secretions that build up behind the cuff, as similarly occurs in VAP patients (28,73). This resulted in localized lung pathology in distinct lobes, as also observed in VAP patients (28,73). However, in this model, the authors did not use a lung-protective ventilation strategy as currently used in patients and animals, where, as discussed above, lungs are typically ventilated with approximately 8 mL/kg tidal volumes accompanied by positive end-expiratory pressure to mitigate end-expiratory alveolar collapse. Perhaps this or less control on etiology leads to more variability and a clinical course of experimental VAP that appears slightly different from that observed in VAP patients (73).
Agar-bead (chronic) pneumonia model
This model is used extensively in the field of cystic fibrosis research and was originally developed in rats (74). To mimic biofilm, agar or seaweed alginate beads are used as extracellular polymeric substances which are loaded with bacteria in a process that requires mixing with mineral oil (55,75) and addition of an emulsifying agent, sorbitan-monooleate, to increase uniformity of the beads (75) (Figure 1). For sham animals, sterile beads are prepared using PBS or saline, however, instillation of sterile beads itself incites an inflammatory response resulting in increased cellular infiltrates in lungs (55,75) (Figure 2C) and increased release of inflammatory cytokines that, depending on the precise clinical endpoints utilized in the study, could obscure the potential beneficial treatment effects (75).
Interestingly, although bacteria can migrate from the agarose beads in vivo, bacterial growth is slow and is limited to the beads, a situation that is similar to what has been observed in bacteria existing in a biofilm phenotype (76). Also, clearance of bacteria is impaired and animals are less likely to develop acute sepsis that occurs more frequently with free-living bacterial inoculation (54). Interestingly, P. aeruginosa loaded agar beads induce several of the main characteristics of the chronic lung infection observed in CF patients including lung histopathology and elevation in lung neutrophils and increased cytokines (22). In particular, rodents, when inoculated with P. aeruginosa loaded beads, highly reproduce human pathology by developing a diffuse bronchopneumonia type of lung histopathology (Figure 2C). This is accompanied by a higher production of proinflammatory cytokines and a more significant weight loss compared to animals receiving free-living bacteria with equal bacterial titers (54). Although the precise reasons for this remains unknown, extensive neutrophil influx in response to the P. aeruginosa-loaded beads has been noted and is proposed to cause severe airway obstruction and limited gas exchange (22), especially in mice (54). Additionally, few studies have shown that mixing free-living bacteria with sterile beads produces similar pathological changes as observed with bacteria-loaded beads (54,77) (Figure 1). However, P. aeruginosa-loaded agarose beads better resemble the chronic lung infection observed in CF patients with regards to histopathological features, elevation in lung neutrophils and the accumulation of cytokines in epithelial lining fluid (54).
Unmet pre-clinical need in pneumonia animal modeling
A better control on etiology and administered dose
Of utmost importance in developing animal models of infection is to accurately estimate the bacterial dose given to each animal and to keep this dose consistent between different independent experiments. Consistent culturing methods along with predetermined standards for optical density measurements that correlate with colony forming unit (CFU) counts is, in that respect, one of the most accurate methods that does not require costly equipment such as flow cytometers. Nonetheless, it is recommended that, for every inoculum used for an experiment, the actual bacterial load be validated using quantitative culture followed by (CFU) counts (54).
Besides a good control on administered dose, the choice of a particular strain can have profound effects on the phenotype of the pneumonia model. For example, naturally occurring P. aeruginosa isolates can lack ExoU expression, an important toxin secreted by the type 3 secretion system (T3SS). These isolates induce a reduced pneumonia phenotype in animal models (78,79). Furthermore, strains isolated from CF patients have reduced T3SS expression (80) while biofilm formation in these strains is enhanced resulting in a mucoid phenotype due to high alginate expression (81). Therefore, mucoid strains like PAO1 should be preferred for developing CF models of chronic pneumonia. However, a precise bacterial count for highly mucoid strains is more difficult and leads to high inter-experiment variance.
An important issue that also needs to be addressed is the fact that different bacterial strains are host-specific (82) and only express certain virulence factors depending on the particular host niche (83). In that respect, clinical isolates might not be ideally suited to establish pneumonia in a rodent model, but, on the other hand, rodent adapted bacterial strains might lack expression of specific key virulence factors and thus provoke an altered immune response (84,85). To circumvent this limitation, studies have frequently resorted to supra-physiological high dose inoculation in animals, using, for example, initial doses ranging from 1E7-1E8 CFU in rats (86-88). This, however, does not resemble the common pathophysiological mechanisms observed in human pneumonia where colonization and micro-aspiration are the main initial events leading to infection. However, to study specific aspects of the disease, i.e., the effect of different pathogenic strains or antibiotic testing, high dose inoculation remains the most used method to create an experimental animal pneumonia model (6).
Disease endpoints for translational studies
In vivo non-invasive disease monitoring
In order to accurately follow-up animal disease progression, detailed non-invasive in vivo monitoring is required. One approach is to simply observe the animals for signs of pain, discomfort or distress (Figure 2D), as has been previously utilized (89). In our experience and also of others (89), pneumonia progression can be categorized into 3 distinct stages each containing signs resembling a specific stage during acute lung inflammation/infection. Stage 1 presents signs of early infection/inflammation in the lung, stage 2 presents distinct signs of severe pneumonia and stage 3 presents signs of severely compromised lung functions, as seen in HAP patients when infection overwhelms the host immune system and patients tend to succumb to the disease. The specific signs from stage 2 or stage 3 could be weighted more than those from stage 1 in order to create a clinical scoring scheme.
Recently, unrestrained whole body lung plethysmography has been shown to be able to monitor progressive pneumonia robustly utilizing tidal volume, breathing frequencies, and enhanced pause (Penh) as measures of lung function (Figure 2E) (90,91). While tidal volume and breathing frequencies are being commonly utilized, Penh can only be used in unrestrained plethysmography if the gas in the plethysmograph is pre-conditioned to body temperature and humidity, however, this lung function parameter as a measurement for airway restriction is much debated (92,93).
Other methods for non-invasive disease monitoring include the use of genetically engineered bioluminescent bacteria (94,95). The Photorhabdus luminescence lux operon is one of the best-studied operons to be utilized as a marker (96-98). Luminescent detection, however, requires sufficient signal emission and therefore highly virulent rodent strains that need to be used in lower doses might not be suited for this model as well as larger animal species that do not allow enough transmitted light to be detected by the camera (95).
Bacterial enumeration
As explained above, accurate bacterial enumeration to estimate bacterial load is of utmost importance in animal infection modeling. This becomes especially important when testing antibacterial compounds in animal models. Quantitative lung bacteriology can be performed on BAL fluid, or on homogenized lung tissue, collected post-euthanasia. Additionally, detection of nucleic acids by PCR can also be used to estimate bacterial lung burden (53), however, these assays will also quantify dead bacteria that might not be directly involved in the infectious process.
Lung histopathology
Besides lung bacteriology, histology is one of the key primary endpoints post- euthanasia (Figure 2C). Different lung pathology scoring schemes have been used previously (98), however, the American Thoracic Society has developed a dedicated lung pathology-scoring scheme for animals (99). Next to standard lung histopathology on H&E stained sections, immunohistochemistry for main cell types of the innate immune system including macrophages and neutrophils can aid in grading pneumonia severity.
Inflammatory parameters
Serum samples taken serially from tail vein can be used to monitor the systemic response against developing pneumonia. Acute phase proteins including CRP, serum amyloid protein A, haptoglobin and α2 macroglobulin (100) as well as proinflammatory cytokines including TNFα, IFNγ, IL-1α, IL-1β and IL-6 can be measured in rodents by antibody based detection methods. While ELISAs are being commonly employed, availability of multiplexing platforms such as Luminex and Mesoscale, allowing simultaneous measurement of different proteins from single sample analysis, are highly beneficial when sample amounts are limited. Post-euthanasia, inflammatory profiles can also be established using lung transcript analysis, however, proper handling of tissue prior to RNA extraction is vital. Also other considerations when targeting lung transcript studies have to be taken in account. For instance, when BAL is being performed, the major proportion of alveolar macrophages as well as neutrophils is washed out from airways that can have a drastic effect on total lung transcript readouts. Also, improper washing of tissue can result in mixed lung/blood causing more difficulty in interpreting results. In our experience, snap-freezing lung tissue in liquid nitrogen allows for both downstream protein and RNA studies and gives more flexibility compared to using RNA protection media.
Humanized models
Although humans and rodents belong to the monophyletic group of Mammalia, considerable differences exist between rodents and humans including their immune system. For instance, mice have fewer circulating neutrophils compared to humans (101), have different Toll-like receptor expression patterns in specific cell subsets (102), and respond differently towards specific chemotactic molecules (102). In addition, IL-10 is believed to have a predominant Th2 anti-inflammatory function in rodents, while in humans, both Th1 (proinflammatory) and Th2 cells can secrete IL-10 and serves as an immunomodulatory cytokine (103). Moreover, activation and proliferation of Th17 cells, a T-cell subset important in defense against Gram-negative bacterial pneumonia (104,105), is predominantly induced by IL-1β, IL-6, and IL-23 in humans compared to IL-6 and TGFβ that are the main drivers of Th17 differentiation in rodents (106). Even within the same species, notable differences occur that can influence experimental outcomes. For example, BALB/c mice are classified as a Th2 responder strain compared to C3H/HeN mice that are Th1 responders (26); and establishment of infection using the same bacterial dose led to higher mortality in the Th2 responding strain (26).
In the last decade, these inherent differences between rodents and humans have most likely contributed to the high number of failures observed in human clinical trials (107-109). For example, one meta-analysis study has shown that in the period 2008–2010, 51% of phase II clinical trials failed due to insufficient efficacy of the compound in human pathology (109). In this context, humanized mouse models that have a closer resemblance to the human immune system could offer great benefits in pre-clinical research by lessening type 1 and type 2 errors made by a wrongful extrapolation of results obtained from common animal models. Humanized mouse models have already been extensively employed in research of cancer and human-specific viruses, such as HIV and herpes (110-112), however, their use in bacterial pneumonia models is not well established. A humanized bacterial pneumonia model could offer significant advantages over wild type animals. A recent study using NSG (NOD scid gamma) background mouse strain grafted with human CD34 cells showed increased susceptibility towards clinical isolate USA300 S. aureus strain compared to wild type animals (113). Furthermore, this study identified specific bacterial toxins that were more effective in the humanized mouse strain (113). This indicates that specific host-pathogen interactions drive pneumonia development and illustrates the importance of humanized animal models to study HAP. Additionally, an adult, immunocompetent humanized HAP animal model that mimics “human” host cytokine response would generate data and mechanisms with more translational value in directly investigating more clinically relevant infectious processes of human pneumonia (114). Besides these potential major advantages of utilizing a humanized immune system to study bacterial pneumonia, the commonly used wild-type animal models described above are currently easier to create and allow for more flexibility in the experimental setup and will therefore remain an important tool to tackle the problems surrounding hospital-acquired pneumonia.
Acknowledgements
Funding: This work was supported by the Flemish Fund for Scientific Research (FWO-G051312N), Flemish Institute for Science and technology (IWT-SBO-140746), and University of Antwerp (GOA-s30729). KB (SB111664) and B’SJ (SB151525) are PhD fellows of IWT/FWO Belgium.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Torres A, Ferrer M, Badia JR. Treatment guidelines and outcomes of hospital-acquired and ventilator-associated pneumonia. Clin Infect Dis 2010;51 Suppl 1:S48-53. [Crossref] [PubMed]
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334-49. [Crossref] [PubMed]
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388-416. [Crossref] [PubMed]
- Jorens PG. Sticking to an Old Definition of Ventilator-Associated Pneumonia Is Not Old-Fashioned. Respir Care 2016;61:390-2. [Crossref] [PubMed]
- Cook DJ, Walter SD, Cook RJ, et al. Incidence of and risk factors for ventilator-associated pneumonia in critically ill patients. Ann Intern Med 1998;129:433-40. [Crossref] [PubMed]
- Luna CM, Sibila O, Agusti C, et al. Animal models of ventilator-associated pneumonia. Eur Respir J 2009;33:182-8. [Crossref] [PubMed]
- Rosenthal VD, Maki DG, Jamulitrat S, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary for 2003-2008, issued June 2009. Am J Infect Control 2010;38:95-104.e2. [Crossref] [PubMed]
- Richards MJ, Edwards JR, Culver DH, et al. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit Care Med 1999;27:887-92. [Crossref] [PubMed]
- Kollef MH. Ventilator-associated pneumonia. A multivariate analysis. JAMA 1993;270:1965-70. [Crossref] [PubMed]
- Markowicz P, Wolff M, Djedaini K, et al. Multicenter prospective study of ventilator-associated pneumonia during acute respiratory distress syndrome. Incidence, prognosis, and risk factors. ARDS Study Group. Am J Respir Crit Care Med 2000;161:1942-8. [Crossref] [PubMed]
- Craven DE, Kunches LM, Kilinsky V, et al. Risk factors for pneumonia and fatality in patients receiving continuous mechanical ventilation. Am Rev Respir Dis 1986;133:792-6. [PubMed]
- Fagon JY, Chastre J, Domart Y, et al. Nosocomial pneumonia in patients receiving continuous mechanical ventilation. Prospective analysis of 52 episodes with use of a protected specimen brush and quantitative culture techniques. Am Rev Respir Dis 1989;139:877-84. [Crossref] [PubMed]
- Fukushi M, Ito T, Oka T, et al. Serial Histopathological Examination of the Lungs of Mice Infected with Influenza A Virus PR8 Strain. PLoS One 2011;6:e21207. [Crossref] [PubMed]
- Barbier F, Andremont A, Wolff M, et al. Hospital-acquired pneumonia and ventilator-associated pneumonia: recent advances in epidemiology and management. Curr Opin Pulm Med 2013;19:216-28. [Crossref] [PubMed]
- Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med 2010;362:1804-13. [Crossref] [PubMed]
- Kollef MH, Chastre J, Fagon JY, et al. Global prospective epidemiologic and surveillance study of ventilator-associated pneumonia due to Pseudomonas aeruginosa. Crit Care Med 2014;42:2178-87. [Crossref] [PubMed]
- Bergen GA, Toney JF. Infection versus colonization in the critical care unit. Crit Care Clin 1998;14:71-90. [Crossref] [PubMed]
- Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med 2003;168:918-51. [Crossref] [PubMed]
- Coutinho HD, Falcao-Silva VS, Goncalves GF. Pulmonary bacterial pathogens in cystic fibrosis patients and antibiotic therapy: a tool for the health workers. Int Arch Med 2008;1:24. [Crossref] [PubMed]
- Cutting GR. Cystic fibrosis genetics: from molecular understanding to clinical application. Nat Rev Genet 2015;16:45-56. [Crossref] [PubMed]
- Kerem B, Rommens JM, Buchanan JA, et al. Identification of the cystic fibrosis gene: genetic analysis. Science 1989;245:1073-80. [Crossref] [PubMed]
- Kukavica-Ibrulj I, Levesque RC. Animal models of chronic lung infection with Pseudomonas aeruginosa: useful tools for cystic fibrosis studies. Lab Anim 2008;42:389-412. [Crossref] [PubMed]
- Moreau-Marquis S, Stanton BA, O'Toole GA. Pseudomonas aeruginosa biofilm formation in the cystic fibrosis airway. Pulm Pharmacol Ther 2008;21:595-9. [Crossref] [PubMed]
- Lewis K. Persister cells, dormancy and infectious disease. Nat Rev Microbiol 2007;5:48-56. [Crossref] [PubMed]
- Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 2002;15:167-93. [Crossref] [PubMed]
- Moser C, Johansen HK, Song Z, et al. Chronic Pseudomonas aeruginosa lung infection is more severe in Th2 responding BALB/c mice compared to Th1 responding C3H/HeN mice. APMIS 1997;105:838-42. [Crossref] [PubMed]
- Hotterbeekx A, Xavier BB, Bielen K, et al. The endotracheal tube microbiome associated with Pseudomonas aeruginosa or Staphylococcus epidermidis. Sci Rep 2016;6:36507. [Crossref] [PubMed]
- Li Bassi G, Rigol M, Marti JD, et al. A novel porcine model of ventilator-associated pneumonia caused by oropharyngeal challenge with Pseudomonas aeruginosa. Anesthesiology 2014;120:1205-15. [Crossref] [PubMed]
- Marquette CH, Wermert D, Wallet F, et al. Characterization of an animal model of ventilator-acquired pneumonia. Chest 1999;115:200-9. [Crossref] [PubMed]
- Wermert D, Marquette CH, Copin MC, et al. Influence of pulmonary bacteriology and histology on the yield of diagnostic procedures in ventilator-acquired pneumonia. Am J Respir Crit Care Med 1998;158:139-47. [Crossref] [PubMed]
- Wu Q, Gui P, Yao S, et al. Expression of β-defensin-3 in lungs of immunocompetent rats with methicillin-resistant Staphylococcus aureus ventilator-associated pneumonia. J Surg Res 2011;169:277-83. [Crossref] [PubMed]
- Lin CY, Zhang H, Cheng KC, et al. Mechanical ventilation may increase susceptibility to the development of bacteremia. Crit Care Med 2003;31:1429-34. [Crossref] [PubMed]
- Evans SE, Tuvim MJ, Zhang J, et al. Host lung gene expression patterns predict infectious etiology in a mouse model of pneumonia. Respir Res 2010;11:101. [Crossref] [PubMed]
- Campbell GD, Coalson JJ, Johanson WG Jr. The effect of bacterial superinfection on lung function after diffuse alveolar damage. Am Rev Respir Dis 1984;129:974-8. [PubMed]
- Crouch TW, Higuchi JH, Coalson JJ, et al. Pathogenesis and prevention of nosocomial pneumonia in a nonhuman primate model of acute respiratory failure. Am Rev Respir Dis 1984;130:502-4. [PubMed]
- Li Bassi G, Zanella A, Cressoni M, et al. Following tracheal intubation, mucus flow is reversed in the semirecumbent position: possible role in the pathogenesis of ventilator-associated pneumonia. Crit Care Med 2008;36:518-25. [Crossref] [PubMed]
- Panigada M, Berra L, Greco G, et al. Bacterial colonization of the respiratory tract following tracheal intubation-effect of gravity: an experimental study. Crit Care Med 2003;31:729-37. [Crossref] [PubMed]
- Moser KM, Maurer J, Jassy L, et al. Sensitivity, specificity, and risk of diagnostic procedures in a canine model of Streptococcus pneumoniae pneumonia. Am Rev Respir Dis 1982;125:436-42. [PubMed]
- Shure D, Moser KM, Konopka R. Transbronchial needle aspiration in the diagnosis of pneumonia in a canine model. Am Rev Respir Dis 1985;131:290-1. [PubMed]
- Bretonnière C, Boutoille D, Caillon J, et al. In vivo efficacy of ceftolozane against Pseudomonas aeruginosa in a rabbit experimental model of pneumonia: comparison with ceftazidime, piperacillin/tazobactam and imipenem. Int J Antimicrob Agents 2014;44:218-21. [Crossref] [PubMed]
- Diep BA, Le VT, Visram ZC, et al. Improved Protection in a Rabbit Model of Community-Associated Methicillin-Resistant Staphylococcus aureus Necrotizing Pneumonia upon Neutralization of Leukocidins in Addition to Alpha-Hemolysin. Antimicrob Agents Chemother 2016;60:6333-40. [Crossref] [PubMed]
- Russell WMS, Burch RL. The principles of humane experimental technique. Michigan: Methuen; 1959.
- Morimoto K, Amano H, Sonoda F, et al. Alveolar macrophages that phagocytose apoptotic neutrophils produce hepatocyte growth factor during bacterial pneumonia in mice. Am J Respir Cell Mol Biol 2001;24:608-15. [Crossref] [PubMed]
- Abe T, Tominaga Y, Kikuchi T, et al. Bacterial pneumonia causes augmented expression of the secretory leukoprotease inhibitor gene in the murine lung. Am J Respir Crit Care Med 1997;156:1235-40. [Crossref] [PubMed]
- Dukelow AM, Weicker S, Karachi TA, et al. Effects of nebulized diethylenetetraamine-NONOate in a mouse model of acute Pseudomonas aeruginosa pneumonia. Chest 2002;122:2127-36. [Crossref] [PubMed]
- Mimoz O, Elhelali N, Léotard S, et al. Treatment of experimental pneumonia in rats caused by a PER-1 extended-spectrum beta-lactamase-producing strain of Pseudomonas aeruginosa. J Antimicrob Chemother 1999;44:91-7. [Crossref] [PubMed]
- Attalah HL, Wu Y, Alaoui-El-Azher M, et al. Induction of type-IIA secretory phospholipase A2 in animal models of acute lung injury. Eur Respir J 2003;21:1040-5. [Crossref] [PubMed]
- Drusano GL, Vanscoy B, Liu W, et al. Saturability of granulocyte kill of Pseudomonas aeruginosa in a murine model of pneumonia. Antimicrob Agents Chemother 2011;55:2693-5. [Crossref] [PubMed]
- Peluso L, de Luca C, Bozza S, et al. Protection against Pseudomonas aeruginosa lung infection in mice by recombinant OprF-pulsed dendritic cell immunization. BMC Microbiol 2010;10:9. [Crossref] [PubMed]
- McConnell KW, McDunn JE, Clark AT, et al. Streptococcus pneumoniae and Pseudomonas aeruginosa pneumonia induce distinct host responses. Crit Care Med 2010;38:223-41. [Crossref] [PubMed]
- Rello J, Afessa B, Anzueto A, et al. Activity of a silver-coated endotracheal tube in preclinical models of ventilator-associated pneumonia and a study after extubation. Crit Care Med 2010;38:1135-40. [Crossref] [PubMed]
- Miller MA, Stabenow JM, Parvathareddy J, et al. Visualization of murine intranasal dosing efficiency using luminescent Francisella tularensis: effect of instillation volume and form of anesthesia. PLoS One 2012;7:e31359. [Crossref] [PubMed]
- Mizgerd JP, Skerrett SJ. Animal models of human pneumonia. Am J Physiol Lung Cell Mol Physiol 2008;294:L387-98. [Crossref] [PubMed]
- van Heeckeren AM, Schluchter MD. Murine models of chronic Pseudomonas aeruginosa lung infection. Lab Anim 2002;36:291-312. [Crossref] [PubMed]
- Lavoie EG, Wangdi T, Kazmierczak BI. Innate immune responses to Pseudomonas aeruginosa infection. Microbes Infect 2011;13:1133-45. [Crossref] [PubMed]
- Lovewell RR, Patankar YR, Berwin B. Mechanisms of phagocytosis and host clearance of Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol 2014;306:L591-603. [Crossref] [PubMed]
- Jorens PG, Richman-Eisenstat JB, Housset BP, et al. Pseudomonas-induced neutrophil recruitment in the dog airway in vivo is mediated in part by IL-8 and inhibited by a leumedin. Eur Respir J 1994;7:1925-31. [PubMed]
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 1998;157:294-323. [Crossref] [PubMed]
- Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 2013;369:2126-36. [Crossref] [PubMed]
- Cabrera-Benitez NE, Laffey JG, Parotto M, et al. Mechanical ventilation-associated lung fibrosis in acute respiratory distress syndrome: a significant contributor to poor outcome. Anesthesiology 2014;121:189-98. [Crossref] [PubMed]
- Frank JA, Parsons PE, Matthay MA. Pathogenetic significance of biological markers of ventilator-associated lung injury in experimental and clinical studies. Chest 2006;130:1906-14. [Crossref] [PubMed]
- Kroon AA, Wang J, Kavanagh BP, et al. Prolonged mechanical ventilation induces cell cycle arrest in newborn rat lung. PLoS One 2011;6:e16910. [Crossref] [PubMed]
- Vaneker M, Halbertsma FJ, van Egmond J, et al. Mechanical ventilation in healthy mice induces reversible pulmonary and systemic cytokine elevation with preserved alveolar integrity: an in vivo model using clinical relevant ventilation settings. Anesthesiology 2007;107:419-26. [Crossref] [PubMed]
- Ding N, Wang F, Xiao H, et al. Mechanical ventilation enhances HMGB1 expression in an LPS-induced lung injury model. PLoS One 2013;8:e74633. [Crossref] [PubMed]
- Plötz FB, Vreugdenhil HA, Slutsky AS, et al. Mechanical ventilation alters the immune response in children without lung pathology. Intensive Care Med 2002;28:486-92. [Crossref] [PubMed]
- Vreugdenhil HA, Heijnen CJ, Plötz FB, et al. Mechanical ventilation of healthy rats suppresses peripheral immune function. Eur Respir J 2004;23:122-8. [Crossref] [PubMed]
- Dhanireddy S, Altemeier WA, Matute-Bello G, et al. Mechanical ventilation induces inflammation, lung injury, and extra-pulmonary organ dysfunction in experimental pneumonia. Lab Invest 2006;86:790-9. [Crossref] [PubMed]
- Uhlig S, Uhlig U. Pharmacological interventions in ventilator-induced lung injury. Trends Pharmacol Sci 2004;25:592-600. [Crossref] [PubMed]
- Tremblay L, Valenza F, Ribeiro SP, et al. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest 1997;99:944-52. [Crossref] [PubMed]
- Nahum A, Hoyt J, Schmitz L, et al. Effect of mechanical ventilation strategy on dissemination of intratracheally instilled Escherichia coli in dogs. Crit Care Med 1997;25:1733-43. [Crossref] [PubMed]
- Verbrugge SJ, Sorm V, van 't Veen A, et al. Lung overinflation without positive end-expiratory pressure promotes bacteremia after experimental Klebsiella pneumoniae inoculation. Intensive Care Med 1998;24:172-7. [Crossref] [PubMed]
- Wagener BM, Pittet JF. A more clinically relevant model of ventilator-associated pneumonia? Anesthesiology 2014;120:1075-7. [Crossref] [PubMed]
- Cash HA, Woods DE, McCullough B, et al. A rat model of chronic respiratory infection with Pseudomonas aeruginosa. Am Rev Respir Dis 1979;119:453-9. [PubMed]
- Growcott EJ, Coulthard A, Amison R, et al. Characterisation of a refined rat model of respiratory infection with Pseudomonas aeruginosa and the effect of ciprofloxacin. J Cyst Fibros 2011;10:166-74. [Crossref] [PubMed]
- Hodgson AE, Nelson SM, Brown MR, et al. A simple in vitro model for growth control of bacterial biofilms. J Appl Bacteriol 1995;79:87-93. [Crossref] [PubMed]
- Starke JR, Edwards MS, Langston C, et al. A mouse model of chronic pulmonary infection with Pseudomonas aeruginosa and Pseudomonas cepacia. Pediatr Res 1987;22:698-702. [Crossref] [PubMed]
- Shaver CM, Hauser AR. Relative contributions of Pseudomonas aeruginosa ExoU, ExoS, and ExoT to virulence in the lung. Infect Immun 2004;72:6969-77. [Crossref] [PubMed]
- Sawa T, Shimizu M, Moriyama K, et al. Association between Pseudomonas aeruginosa type III secretion, antibiotic resistance, and clinical outcome: a review. Crit Care 2014;18:668. [Crossref] [PubMed]
- Jain M, Ramirez D, Seshadri R, et al. Type III secretion phenotypes of Pseudomonas aeruginosa strains change during infection of individuals with cystic fibrosis. J Clin Microbiol 2004;42:5229-37. [Crossref] [PubMed]
- Høiby N, Ciofu O, Bjarnsholt T. Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol 2010;5:1663-74. [Crossref] [PubMed]
- Holtfreter S, Radcliff FJ, Grumann D, et al. Characterization of a mouse-adapted Staphylococcus aureus strain. PLoS One 2013;8:e71142. [Crossref] [PubMed]
- Lenski RE, Travisano M. Dynamics of adaptation and diversification: a 10,000-generation experiment with bacterial populations. Proc Natl Acad Sci U S A 1994;91:6808-14. [Crossref] [PubMed]
- Rashid RA, Tabata TA, Oatley MJ, et al. Expression of putative virulence factors of Escherichia coli O157:H7 differs in bovine and human infections. Infect Immun 2006;74:4142-8. [Crossref] [PubMed]
- Casadevall A, Pirofski L. Host-pathogen interactions: the attributes of virulence. J Infect Dis 2001;184:337-44. [Crossref] [PubMed]
- Yaghi A, Bradbury JA, Zeldin DC, et al. Pulmonary cytochrome P-450 2J4 is reduced in a rat model of acute Pseudomonas pneumonia. Am J Physiol Lung Cell Mol Physiol 2003;285:L1099-105. [Crossref] [PubMed]
- Vanderzwan J, McCaig L, Mehta S, et al. Characterizing alterations in the pulmonary surfactant system in a rat model of Pseudomonas aeruginosa pneumonia. Eur Respir J 1998;12:1388-96. [Crossref] [PubMed]
- Webert KE, Vanderzwan J, Duggan M, et al. Effects of inhaled nitric oxide in a rat model of Pseudomonas aeruginosa pneumonia. Crit Care Med 2000;28:2397-405. [Crossref] [PubMed]
- Harris G, Kuo Lee R, Lam CK, et al. A mouse model of Acinetobacter baumannii-associated pneumonia using a clinically isolated hypervirulent strain. Antimicrob Agents Chemother 2013;57:3601-13. [Crossref] [PubMed]
- Bondue B, Vosters O, de Nadai P, et al. ChemR23 dampens lung inflammation and enhances anti-viral immunity in a mouse model of acute viral pneumonia. PLoS Pathog 2011;7:e1002358. [Crossref] [PubMed]
- Julander JG, Kesler K, Van Wettere AJ, et al. The use of plethysmography in determining the severity of lung pathology in a mouse model of minimally lethal influenza virus infection. Antiviral Res 2014;108:10-3. [Crossref] [PubMed]
- Lundblad LK, Irvin CG, Hantos Z, et al. Penh is not a measure of airway resistance! Eur Respir J 2007;30:805. [Crossref] [PubMed]
- Bates J, Irvin C, Brusasco V, et al. The use and misuse of Penh in animal models of lung disease. Am J Respir Cell Mol Biol 2004;31:373-4. [Crossref] [PubMed]
- Xiong YQ, Willard J, Kadurugamuwa JL, et al. Real-time in vivo bioluminescent imaging for evaluating the efficacy of antibiotics in a rat Staphylococcus aureus endocarditis model. Antimicrob Agents Chemother 2005;49:380-7. [Crossref] [PubMed]
- Munder A, Wolbeling F, Klockgether J, et al. In vivo imaging of bioluminescent Pseudomonas aeruginosa in an acute murine airway infection model. Pathog Dis 2014;72:74-7. [Crossref] [PubMed]
- Francis KP, Yu J, Bellinger-Kawahara C, et al. Visualizing pneumococcal infections in the lungs of live mice using bioluminescent Streptococcus pneumoniae transformed with a novel gram-positive lux transposon. Infect Immun 2001;69:3350-8. [Crossref] [PubMed]
- Francis KP, Joh D, Bellinger-Kawahara C, et al. Monitoring bioluminescent Staphylococcus aureus infections in living mice using a novel luxABCDE construct. Infect Immun 2000;68:3594-600. [Crossref] [PubMed]
- Rouby JJ, Martin De Lassale E, Poete P, et al. Nosocomial bronchopneumonia in the critically ill. Histologic and bacteriologic aspects. Am Rev Respir Dis 1992;146:1059-66. [Crossref] [PubMed]
- Matute-Bello G, Downey G, Moore BB, et al. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol 2011;44:725-38. [Crossref] [PubMed]
- Cray C, Zaias J, Altman NH. Acute phase response in animals: a review. Comp Med 2009;59:517-26. [PubMed]
- Doeing DC, Borowicz JL, Crockett ET. Gender dimorphism in differential peripheral blood leukocyte counts in mice using cardiac, tail, foot, and saphenous vein puncture methods. BMC Clin Pathol 2003;3:3. [Crossref] [PubMed]
- Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol 2004;172:2731-8. [Crossref] [PubMed]
- Del Prete G, De Carli M, Almerigogna F, et al. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol 1993;150:353-60. [PubMed]
- Xu X, Shao B, Wang R, et al. Role of Interleukin-17 in defense against pseudomonas aeruginosa infection in lungs. Int J Clin Exp Med 2014;7:809-16. [PubMed]
- Kolls JK, McCray PB Jr, Chan YR. Cytokine-mediated regulation of antimicrobial proteins. Nat Rev Immunol 2008;8:829-35. [Crossref] [PubMed]
- Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, et al. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol 2007;8:942-9. [Crossref] [PubMed]
- Green AR. Why do neuroprotective drugs that are so promising in animals fail in the clinic? An industry perspective. Clin Exp Pharmacol Physiol 2002;29:1030-4. [Crossref] [PubMed]
- Rohra DK, Jawaid A. Reliability of rodent animal models in biomedical research. J Coll Physicians Surg Pak 2005;15:809-12. [PubMed]
- Arrowsmith J. Trial watch: Phase II failures: 2008-2010. Nat Rev Drug Discov 2011;10:328-9. [Crossref] [PubMed]
- Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol 2007;7:118-30. [Crossref] [PubMed]
- Zhang L, Kovalev GI, Su L. HIV-1 infection and pathogenesis in a novel humanized mouse model. Blood 2007;109:2978-81. [PubMed]
- Shultz LD, Saito Y, Najima Y, et al. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2r gamma(null) humanized mice. Proc Natl Acad Sci U S A 2010;107:13022-7. [Crossref] [PubMed]
- Prince A, Wang H, Kitur K, et al. Humanized mice exhibit increased susceptibility to Staphylococcus aureus pneumonia. J Infect Dis 2016.jiw425. [Crossref] [PubMed]
- Brehm MA, Wiles MV, Greiner DL, et al. Generation of improved humanized mouse models for human infectious diseases. J Immunol Methods 2014;410:3-17. [Crossref] [PubMed]


