Use of distal perfusion in peripheral extracorporeal membrane oxygenation
Introduction
Extra corporeal membrane oxygenation (ECMO) indications and usage has strikingly progressed over the last 20 years; it has become essential tool in the care of adults and children with severe cardiac and pulmonary dysfunction refractory to conventional management (1-4). According to the Extracorporeal Life Support Organization (ESLO) registry, extracorporeal life support (ECLS) was used in over 7,900 cases in 2015 (5).
Femoral access is preferred for venoarterial ECMO (VA ECMO) in case of emergency or cardiogenic shock as the insertion is relatively less invasive and faster to institute the ECMO. It may be performed bedside in the intensive care unit, emergency department or cardiac catheterization lab. It also can be performed while the rapid response team (RRT) is performing cardiopulmonary resuscitation (CPR) (3,4). It was also used in patients with cardiogenic shock in hospital without on-site cardiac surgical facilities (6). In addition to other known ECMO complications, it has its own specific complications as upper body hypoxemia and ischemia of the ipsilateral lower extremity (4). Here we will focus on the published data of this complication, outline the techniques (Figures 1-3) used to prevent this complication which may lead to limb loss and markedly impact the patient’s quality of life.
Discussion
Vascular complications in femoral ECMO
A variety of vascular complications can occur in patients undergoing femoral VA ECMO, Most common causes are listed in Table 1. These complications occur during cannulation, including vessel perforation with hemorrhage, arterial dissection, distal ischemia, and incorrect location (e.g., venous cannula within the artery) or development of pseudo-aneurysm at the site of insertion. Complications may also occur during the ECMO support or while performing ECMO separation (4,7-10). Compartment syndrome and lower leg ischemia should be closely observed: this might occur not only form the decrease of blood supply but it might also occur from limb hypoperfusion (11). Patient’s condition and co-morbidities play a major role as well as medications and length of support. In patients with history of peripheral vascular disease, femoral cannulation should be avoided, albeit this is often difficult to find this documentation in a patient in cardiogenic shock and in active CPR. Diabetes also was identified as an independent factor which leads to worse outcomes (12,13).
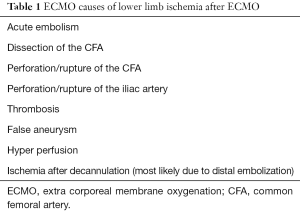
Full table
Cheng et al. (14) in their meta-analysis literature review of 1866 cases of ECMO applied for cardiogenic shock reported lower extremity ischemia occurring in 16.9% (range, 12.5–22.6%),compartment syndrome and fasciotomy accounted for 10.3% (range, 7.3–14.5%), and lower extremity amputation was required in 4.7% (range, 2.3–9.3%). Similar results were reported by von Segesser et al. (15) in their literature review, with vascular complications ranging between 11% and 52%, rate of surgical intervention ranging between 9% and 22%, and leg amputation rate ranging from 2% to 10%. Aziz et al. (13) reported 18% of the patients experienced vascular complications - among these, 16% needed surgical intervention.
Although some papers suggested that leg ischemia doesn’t correlate with the ECMO outcomes (9), recent papers noted that vascular complications were associated with unsuccessful weaning of ECMO (16), and leg ischemia is an independent risk factor for in-hospital death (17). However, amputation inevitably impacts on patient quality of life.
Techniques used to optimize the distal limb perfusion
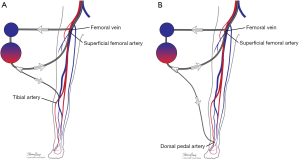
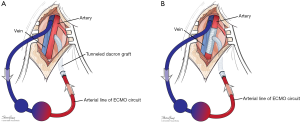
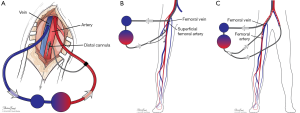
Many maneuvers were made to decrease the incidence of distal ischemia by avoiding inserting the arterial cannula directly into the artery lumen by using end-to-side chimney graft (18,19) or T graft (20,21), these are demonstrated in Figure 1. With this technique, the cannula avoids obstructing the artery. This also allows tunneling the graft to exit in separate incision which may decrease infections. Another approach is by providing blood infusion through distal perfusion cannula (DPC): this may be either antegrade or retrograde perfusion. The DPC is mainly percutaneously inserted, but it can be performed in open technique with direct vessel cut-down (Figure 2A). Different parts of distal limb arterial tree can be used as cannulation sites, including common femoral artery (10), superficial femoral artery (SFA) (8) (Figure 2B), cannulation of the posterior tibial with retrograde perfusion (7) and dorsalis pedis (22) (Figure 3A,B). The contralateral femoral artery can be used also: the DPC inserted through the contralateral femoral artery and is directed into the ipsilateral femoral artery distal to the cannula (Figure 2C). For example, this approach was utilized in a case of a 19-year-old woman who was admitted with cardiogenic shock: after attempts to put DPC prior to ECMO initiation failed, the patient was placed on ECMO support, stabilized, and then the DPC was inserted by the interventional radiologist with satisfactory distal perfusion.
DPC evidence based
Lamb et al. (23) reported a series of 91 patients supported with VA-ECMO with femoral arterial cannula. Percutaneous DPC was placed immediately in 62 of 91 patients, with no subsequent ischemia in these patients. Of the 29 patients without initial DPC placement, 12 (41.4%) developed acute ischemia. Bisdas et al. (9) reported 10% of their series experienced vascular complications. All patients received antegrade distal limb perfusion with the venoarterial access used in 143 patients (82%), and venovenous in 31 patients (18%). Of the 17 (10%) observed vascular complications, 15 (88%) occurred in patients with venoarterial access, Two patients who had extremity ischemia required limb amputation. It has been suggested that measuring the mean pressure in the SFA and inserting a shunt when mean pressure is less than 50 mmHg (24).
Timing of insertion
The best time is during ECMO insertion, in our institution we find easier to insert the distal arterial wire first, followed by percutaneous arterial and venous cannulation, then distal cannula insertion. In all cases the sooner the distal perfusion is initiated, the better. Spurlock et al. (7) reported using distal limb perfusion in 36 patients through posterior tibial artery in retrograde fashion all patient underwent distal perfusion in less than 6 hours of ECMO initiation time didn’t have any ischemic limb injury compare to 20% of patient more than 6 hours.
Complications of DPC
Distal perfusion is not a perfect solution, as it also has it is own complications like occlusion and limb ischemia due to the small catheter size and the inability to control the amount of flow inside which might lead to the other complication with compartment syndrome due to hyper-perfusion in the distal limb (6).
Tips to reduce risk of vascular complications
Peripheral VA ECMO should be avoided in a patient with a history of peripheral vascular disease. If Seldinger technique is used, avoid placing the arterial and venous cannula on the same side in order to avoid venous congestion from compression of the femoral vein by the adjacent arterial cannula. The best way to decrease distal ischemia is to insert the arterial cannula into an end-to-side graft or inserted a DPC to perfuse the distal extremity at the time of ECMO insertion. The earlier the intervention, the better: don’t wait for compartment syndrome to develop to perform fasciotomy. Involvement of a vascular surgeon in ECMO separation may be indicated as approximate 30% require some vascular reconstruction (25).
In summary
Compartment syndrome and lower leg ischemia occur in femoral cannulation. It can result in hypo- or hyper-perfusion to the distal limb and should be closely observed. Options to lower the risk of vascular complications with arterial cannula placement may include locating the arterial cannula contralateral to the venous cannula, use of a vascular conduit, and the use distal perfusion catheter, which is one of the best solution. Ideally, DPC should be inserted at the time of ECMO initiation or shortly after. A multidisciplinary approach to arterial decannulation for vascular reconstruction is recommended.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- MacLaren G, Combes A, Bartlett RH. Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med 2012;38:210-20. [Crossref] [PubMed]
- Shekar K, Mullany DV, Thomson B, et al. Extracorporeal life support devices and strategies for management of acute cardiorespiratory failure in adult patients: a comprehensive review. Crit Care 2014;18:219. [Crossref] [PubMed]
- Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63. [Crossref] [PubMed]
- Makdisi G, Wang IW. Extra corporeal membrane oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis 2015;7:E166-76. [PubMed]
- Extracorporeal Life Support Registry Report international summary. Available online: https://www.elso.org/Registry/Statistics/InternationalSummary.aspx. Accessed on August 8, 2016.
- Belle L, Mangin L, Bonnet H, et al. Emergency extracorporeal membrane oxygenation in a hospital without on-site cardiac surgical facilities. EuroIntervention 2012;8:375-82. [Crossref] [PubMed]
- Spurlock DJ, Toomasian JM, Romano MA, et al. A simple technique to prevent limb ischemia during veno-arterial ECMO using the femoral artery: the posterior tibial approach. Perfusion 2012;27:141-5. [Crossref] [PubMed]
- Roussel A, Al-Attar N, Alkhoder S, et al. Outcomes of percutaneous femoral cannulation for venoarterial extracorporeal membrane oxygenation support. Eur Heart J Acute Cardiovasc Care 2012;1:111-4. [Crossref] [PubMed]
- Bisdas T, Beutel G, Warnecke G, et al. Vascular complications in patients undergoing femoral cannulation for extracorporeal membrane oxygenation support. Ann Thorac Surg 2011;92:626-31. [Crossref] [PubMed]
- Rao AS, Pellegrini RV, Speziali G, et al. A novel percutaneous solution to limb ischemia due to arterial occlusion from a femoral artery ECMO cannula. J Endovasc Ther 2010;17:51-4. [Crossref] [PubMed]
- Mosquera VX. Solla-Buceta M2, Pradas-Irún C3, Lower limb overflow syndrome in extracorporeal membrane oxygenation. Interact Cardiovasc Thorac Surg 2014;19:532-4. [Crossref] [PubMed]
- Rastan AJ, Dege A, Mohr M, et al. Early and late outcomes of 517 consecutive adult patients treated with extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. J Thorac Cardiovasc Surg 2010;139:302-11, 311.e1.
- Aziz F, Brehm CE, El-Banyosy A, et al. Arterial complications in patients undergoing extracorporeal membrane oxygenation via femoral cannulation. Ann Vasc Surg 2014;28:178-83. [Crossref] [PubMed]
- Cheng R, Hachamovitch R, Kittleson M, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg 2014;97:610-6. [Crossref] [PubMed]
- von Segesser L, Marinakis S, Berdajs D, et al. Prevention and therapy of leg ischaemia in extracorporeal life support and extracorporeal membrane oxygenation with peripheral cannulation. Swiss Med Wkly 2016;146:w14304. [PubMed]
- Aubron C, Cheng AC, Pilcher D, et al. Factors associated with outcomes of patients on extracorporeal membrane oxygenation support: a 5-year cohort study. Crit Care 2013;17:R73. [Crossref] [PubMed]
- Hei F, Lou S, Li J, et al. Five-year results of 121 consecutive patients treated with extracorporeal membrane oxygenation at Fu Wai Hospital. Artif Organs 2011;35:572-8. [Crossref] [PubMed]
- Foley PJ, Morris RJ, Woo EY, et al. Limb ischemia during femoral cannulation for cardiopulmonary support. J Vasc Surg 2010;52:850-3. [Crossref] [PubMed]
- Read R, St Cyr J, Tornabene S, et al. Improved cannulation method for extracorporeal membrane oxygenation. Ann Thorac Surg 1990;50:670-1. [Crossref] [PubMed]
- Calderon D, El-Banayosy A, Koerner MM, et al. Modified T-Graft for Extracorporeal Membrane Oxygenation in a Patient with Small-Caliber Femoral Arteries. Tex Heart Inst J 2015;42:537-9. [Crossref] [PubMed]
- Jackson KW, Timpa J, McIlwain RB, et al. Side-arm grafts for femoral extracorporeal membrane oxygenation cannulation. Ann Thorac Surg 2012;94:e111-2. [Crossref] [PubMed]
- Kimura N, Kawahito K, Ito S, Murata S, et al. Perfusion through the dorsalis pedis artery for acute limb ischemia secondary to an occlusive arterial cannula during percutaneous cardiopulmonary support. J Artif Organs 2005;8:206-9. [Crossref] [PubMed]
- Lamb KM, DiMuzio P, Moudgill N, et al. Fate of the Lower Extremity In Patients After VA-ECMO Via Femoral Cannulations: Limb Salvage Protocol Can Decrease Ischemic Complications. JVS 2015;61:190S-191S.
- Chen YS, Lin JW, Yu HY, et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet 2008;372:554-61. [Crossref] [PubMed]
- Wagner AM, Phair J, Scher L, et al. Vascular Complications Associated With Extracorporeal Membrane Oxygenation. JVS 2015;62:791-2.