Emerging treatments for hemophilia: patients and their treaters spoilt for choice, but laboratories face a difficult path?
Hemophilia defines a condition that predisposes to bleeding, and is caused by deficiency or defect in certain clotting factors, the most common being Hemophilia A [factor VIII (FVIII) deficiency] and Hemophilia B [factor IX (FIX) deficiency]. The current treatment for patients with Hemophilia, so that patients are less likely to bleed, entails replacement of the missing factor(s). This was achieved historically using factor concentrates derived from human donor blood plasma, and later using recombinant technology. There have been several advances in this field. Initial full length recombinant products were “replaced” with truncated products, conceived to have similar efficacy but otherwise be more advantageous for patients. This included the first modified FVIII product introduced, namely B-domain deleted (BDD) recombinant FVIII. More recent efforts have focused on modifications to factor proteins in order to extend their half-life and so decreasing the frequency of injections needed for therapy, without concomitantly increasing the risk of bleeding (1-4). The new long-acting therapeutic replacement products include various modifications, such as fusion with Fc region of IgG1 immunoglobulin (-Fc) or albumin, or non-specific or site-specific attachment of polyethylene glycol (PEG) of different molecular weights.
Monitoring of hemophilic patients on therapy is undertaken for many situations, such as occasional monitoring of prophylactic therapy, assessment of pre- and post-surgical factor levels, and investigation of inhibitor status (1,5-7). In most laboratories, factor levels are assessed using simple clot-based assays. For FVIII and FIX, for example, this would involve modified activated partial thromboplastin time (APTT) assays, using mixtures of normal and patient plasma in the presence of factor deficient plasma, and a reference plasma with a pre-defined level of factor FVIII and FIX. Less commonly employed are chromogenic assays (7,8). These assays perform reasonably well when assessing native human derived FVIII or FIX, or full length recombinant products. Some concern with some truncated forms or recombinant protein arose with BDD FVIII, such that assay results could become inaccurate using human FVIII-based reference plasma. The supposed “fix” for this event required the use of a different reference standard. Nevertheless, this then meant that laboratories had to perform different FVIII assays for different purposes, instead of a single FVIII assay for all purposes. This situation is potentially going to get much more complex and costly for laboratories to manage. In brief, each new therapeutic “advance” is progressed according to clinical efficacy, and the laboratory aspects of monitoring treated patients is an after-thought for the companies producing, and potentially also the clinicians prescribing, these products.
These newly emerging products, now often termed extended life products (ELP) will likely be of true benefit to patients, because they provide similar efficacy for reduced interventions. For FVIII, the reduction in the number of injections is likely to be moderate, as these ELP have limited “additional life”, perhaps reducing 3–4/week injections to 2–3/week in order to achieve the same efficacy. For FIX, the reduction in the number of injections is likely to be larger, with the potential to reducing therapy to 1–2/week injections.
Several aspects influence test results of factor activity. One-stage APTT based clotting assays comprise a wide variety of APTT reagents, which differ in phospholipid source (synthetic or extract from plant or animal), phospholipid type and concentration, as well as activator type (e.g., ellagic acid, cellite, kaolin, silica of various types, polyphenols). The factor-deficient plasma used in the assay may either be sourced from congenitally deficient Hemophilia A or B patients, or more commonly after targeted removal of either FVIII or FIX from normal pooled plasma using immuno-depletion, which in some cases of FVIII depletion may also remove von Willebrand factor (VWF), an important protein for safeguarding FVIII integrity in blood. Finally, the analyzer used (i.e., mechanical vs. optical clot detection), the assay protocol/setup used (e.g., single, dual or hybrid calibration curves with or without extrapolation) may also influence test results. Despite this, one-stage factor assays with native human FVIII and FIX provide acceptable reproducibility, largely irrespective of these elements (7).
This is not so with the newly emerging ELP. With many of these products wide variation in results has been observed between different one stage factor clotting assays, as well as between these and chromogenic factor assays, so leading to substantial over- or under-estimation of factor level and potential for inappropriate patient management based on inaccurate test results. In most cases, over- or under-estimation is upwards of around 50%. However, in some cases, the differences are staggering, with measured activity up to 20 or 30 times higher than expected results based on product labeled potency (9,10). Such overestimation is believed to be related to a silica-mediated premature activation of pegylated FIX, which leads to massive activated FIX (FIXa) generation during the contact activation phase of the one-stage FIX activity assay (prior to the re-calcification step).
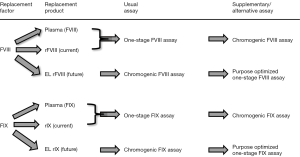
In conclusion, the current typical scenario of the common one-stage FVIII and one-stage FIX assays, occasionally supplemented by a chromogenic FVIII assay, is bound to require a big rethink for hemostasis laboratories that help to manage hemophilia A and B patients under therapy (Figure 1). This impending change is especially significant for generalized laboratories, in which second- and third-line hemostasis testing is only a part of the broad panel of tests performed. One possible proposed solution is to perform chromogenic FVIII and FIX assays in the future for such patients, as these seem less variably affected by the different ELP (11). The alternative, to perform different one-stage FVIII and FIX assays optimized for each ELP (i.e., with specifically selected reference material, APTT reagent and factor deficient plasma), will generate an unreasonable organizational and economic burden on laboratories, especially if the local regulatory authority clears multiple ELP to market in different geographies, more or less as for assessment of direct oral anticoagulants (DOACs) (12). In the new world of hemophilia treatment, patients and their treaters will indeed be spoilt for choice, but laboratories will face a very difficult path, and again, reflecting an added cost impost on cash-strapped clinical laboratories (13,14).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Coppola A, Marrone E, Conca P, et al. Safety of Switching Factor VIII Products in the Era of Evolving Concentrates: Myths and Facts. Semin Thromb Hemost 2016;42:563-76. [Crossref] [PubMed]
- Kumar R, Dunn A, Carcao M.. Changing Paradigm of Hemophilia Management: Extended Half-Life Factor Concentrates and Gene Therapy. Semin Thromb Hemost 2016;42:18-29. [Crossref] [PubMed]
- Giangrande P.. The Future of Hemophilia Treatment: Longer-Acting Factor Concentrates versus Gene Therapy. Semin Thromb Hemost 2016;42:513-7. [Crossref] [PubMed]
- Berntorp E, Andersson NG. Prophylaxis for Hemophilia in the Era of Extended Half-Life Factor VIII/Factor IX Products. Semin Thromb Hemost 2016;42:518-25. [Crossref] [PubMed]
- Fischer K, Berntorp E.. Targeting Factor Replacement Therapy in Severe Hemophilia: Which Level Is Important? Semin Thromb Hemost 2015;41:860-3. [Crossref] [PubMed]
- Carcao MD, Iorio A. Individualizing Factor Replacement Therapy in Severe Hemophilia. Semin Thromb Hemost 2015;41:864-71. [Crossref] [PubMed]
- Favaloro EJ, Meijer P, Jennings I, et al. Problems and Solutions in Laboratory Testing For Hemophilia. Semin Thromb Hemost 2013;39:816-33. [Crossref] [PubMed]
- Lippi G, Franchini M, Favaloro EJ. One-stage clotting versus chromogenic assays for assessing recombinant factor VIII: two faces of a haemostasis coin. Blood Coagul Fibrinolysis 2009;20:1-3. [Crossref] [PubMed]
- Bowyer AE, Hillarp A, Ezban M, et al. Measuring IX activity of nonacog beta pegol with commercially-available one-stage clotting and chromogenic assay kits: A two-center study. J Thromb Haemost 2016;14:1428-35. [Crossref] [PubMed]
- Hubbard AR. Potency Labeling of Novel Factor VIII and Factor IX Concentrates: Past Experience and Current Strategy. Semin Thromb Hemost 2015;41:849-54. [Crossref] [PubMed]
- Kitchen S, Tiefenbacher S, Gosselin R.. Factor Activity Assays for Monitoring Extended Half Life FVIII and Factor IX Replacement Therapies. Semin Thromb Hemost 2017. [Epub ahead of print]. [PubMed]
- Lippi G, Favaloro EJ. Laboratory monitoring of direct oral anticoagulants (DOACs). The perfect storm? Ann Transl Med 2017;5:6. [Crossref] [PubMed]
- Lippi G, Bassi A, Bovo C. The future of laboratory medicine in the era of precision medicine. J Lab Precis Med 2016;1:7. [Crossref]
- Lippi G, Plebani M.. Laboratory economics. Risk or opportunity? Clin Chem Lab Med 2016;54:1701-3. [Crossref] [PubMed]


