Bioaerosols in the lungs of subjects with different ages—Part 2: clearance modeling
Introduction
General remarks on particle clearance in the human respiratory tract
As already outlined in part 1 of the study (1), bioaerosol particles deposited in different structures of the human respiratory tract may act as triggers of allergic reactions, infectious diseases or, in the worst case, malignant transformations. In order to reduce the hazardous potential of such particulate substances, an innate defense system consisting of different clearance phases is activated immediately after bioaerosol inhalation (2-7). Whilst biogenic particles undergoing deposition in the air-conducting zone are subjected to fast and slow bronchial clearance mechanisms, those particulate masses accumulated in the gas-exchange zone are usually seized by alveolar clearance mechanisms, which are also marked by variable velocities (Figure 1) (8-12). As demonstrated by numerous inhalation experiments, fast bronchial clearance, being represented by the so-called ‘mucociliary escalator’, mainly takes place within the first 24 h following particle exposure (13-15). Slow bronchial clearance, on the other hand, may reach a length of up to 100 d, because it partly consists of very protracted processes such as particle transfer towards lung-associated lymph nodes or particle detection and uptake by airway macrophages (1,16-18). Particles deposited in the alveolar structures are primarily phagocytosed by macrophages immigrating into the compartments of gas exchange from subepithelial tissue layers. The particle-loaded cells either migrate into the tracheobronchial tree, from where they are finally removed by coughing or swallowing, or turn back to the alveolar interstitium (1,10,19). This rather fast mechanism of alveolar clearance, however, is accompanied by much slower ones, which concern all those particles being too large or too small for uptake by macrophages. In this specific case clearance by epithelial endocytosis and subsequent transcytosis as well as particle movement on the surfactant blanket occur as alternative mechanisms (1,10,19,20). As found by experimental and theoretical investigations, all clearance processes noted above depend upon both size and shape of the deposited particles (4-12).
Theoretical models of particle clearance—state of the art
The theoretical study of particle clearance in the human respiratory tract looks back on a nearly fifty-year-old tradition (20,21). Whilst first mathematical approaches to the clearance process were mainly limited to the description of fast bronchial clearance (22,23), later models also included essential clearance mechanisms being marked by much longer half-times (4-12,19). In the meantime, different model types predicting the various paths of particle transport have been established and validated by related experimental lines. Among these approaches, the stochastic model formerly outlined by Hofmann & Sturm (6) as well as the comprehensive multi-compartment model introduced by Sturm & Hofmann (5,7,8) deserve particular mention due to their comprehensive application and improvement during the past decade (24-31). Current theoretical research includes the further specification of possible clearance routes and associated clearance times. In addition, modern clearance models are used for the creation of well-aimed experiments, which should help to get a better insight into the particle-related processes of the lungs.
Main objectives pursued by the present contribution
Based upon the deposition data presented in part 1 of the study (1), the contribution tries to work out main differences of biogenic particle clearance among the age groups (1, 5, 15, 20 y) already introduced previously. With reference to the deposition calculations presented in the first part of the investigation it is hypothesized that also particle clearance exhibits a partly significant age dependence, which might have remarkable consequences in pulmonological regards.
Methods
Main features of the applied clearance model
Predictions of bioparticle clearance in the lungs of probands with different ages were conducted by using the stochastic model of particle removal in the bronchial and alveolar structures of the human respiratory tract. Since this approach has been outlined in detail in numerous previous publications (4-7,11,12,21), only its most salient features will be described here. In correspondence with the experimental and theoretical results stated in the introduction, the model considers four main clearance phases: besides fast and slow bronchial clearance mainly seizing all those particles deposited in the ciliated airways (4-7) also fast and slow alveolar clearance are distinguished (10,18,19,24), with the latter two phases playing an essential role in the non-ciliated alveolar ducts and alveoli themselves (Figure 2).
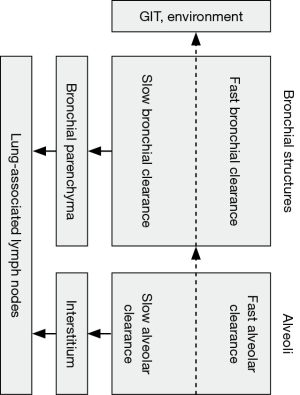
Fast bronchial clearance is founded upon the hypothesis that predefined fractions of deposited particles are captured on a mucus blanket lining the bronchial epithelium. This mucus layer is transported from small ciliated airways towards the trachea, where it is coughed up or swallowed. Mucus velocities are thought to correlate with airway morphometry on the one hand and the intensity of local mucus production or the occurrence of mucus discontinuities on the other (4-12,19). Whilst mucus velocity is generally subject to an exponential decrease from trachea to small ciliated airways situated at the lung periphery, local addition of mucus from goblet cells and subepithelial glands results in an increase of the associated velocity. Local mucus discontinuities cause a remarkable reduction of the mucus mass and, as a consequence of that, a decrease of the related velocity (7,12,30). The fraction of particles being subjected to slow bronchial clearance depends on both particle size and shape. Therefore, large particles have a lower chance to undergo slow clearance than small particles (4-12). According to the recommendations of Sturm & Hofmann (19) extremely anisometric particles such as long fibers or thin platelets with high projective diameters are also preferential targets of fast bronchial clearance, whereas spherical particles may be characterized by an enhanced probability of slow clearance. The half-time of slow clearance is supposed to adopt values between 5 and 20 d, resulting in a total duration of this phase between 25 and 100 d (3-8,20).
Fast alveolar clearance represented by the phagocytosis of biogenic particles is marked by half-times, which are very similar to those of slow bronchial clearance (19,20,30). Here, migration of particle-loaded macrophages through the tracheobronchial tree is assumed to take place within a shorter period of time than interstitial migration of the cells and subsequent transfer of particulate mass into the lymphatic system. Computation of the fraction of bioparticles undergoing slow alveolar clearance is based upon similar considerations as the estimation of the slow bronchial clearance fraction. Therefore, macrophages are thought to preferably take up those particles ranging in size from 1 to 10 µm, whereas smaller or larger particles are mostly excluded from the fast alveolar clearance phase (19,30). Half-time of slow alveolar clearance has been pledged to 100 d, resulting in a total duration of this phase of about 500 d (10,19,30).
Theoretical effect of the probands’ age on particle clearance
As already demonstrated in previous publications (3,11,25), tracheal mucus velocity represents a physiological determinant depending upon subject’s age. In order to make allowance for this essential circumstance, tracheal mucus velocity was assumed to adopt a mean value of 1.20 mm/min in infants (1 y), 2.70 mm/min in children (5 y), 5.00 mm/min in adolescents (15 y), and 5.50 mm/min in adults (20 y). According to the modeling procedure described above, mucus velocities in airways of following lung generations are mathematically related to tracheal mucus velocity, so that predicted velocities in infants assume significantly lower values than those in adults. Although fast bronchial clearance is characterized by valuable differences between the age groups, all other clearance phases are thought to take place in uniform, age-independent fashion. Summing up, clearance efficiency of bioparticles is influenced by the deposition patterns already predicted in the first part of the study (1), the morphometry of the tracheobronchial tree, the velocity of mucociliary transport, and the duration of the remaining clearance phases (Figure 2).
Results
Retention of bioparticles in the respiratory tract
According to numerous previous publications (4-12,19), an appropriate measure of clearance efficiency is represented by the so-called retention value, which specifies the percentage of particles still being stored in the lung structures after a given period of time. In the concrete case, 24-h-retention indicating the temporal course of fast bronchial clearance was distinguished from 25-d-retention und 100-d-retention, both acting as indicators for slow clearance processes. For all age groups considered here, respective retention values were expressed as functions of particle size (Figure 3). Starting with infants (1 y), all retention curves exhibit a unimodal size dependence, with highest values being noticeable for aerodynamic particle diameters ranging from 0.01 to 1.0 µm. For smaller and larger particles, a remarkable reduction of bioparticle retention may be observed. As illustrated in the related graph, 24-h-retention commonly ranges from 5.4% to 76.3%, whereas 25-d-retention assumes values between 0.0 and 10.1%. Particle retention after 100 d is marked by values ranging from 0.0 to 4.8% (Figure 3A).
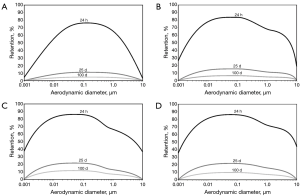
In children (5 y) predicted retention curves are subject to a substantial widening on the one side and a significant displacement to higher values on the other. In terms of fact, 24-h-retention is characterized by values ranging from 20.2 to 83.6%, with maximum values being identifiable for particle diameters between 0.01 and 0.1 µm. Particle retention after 25 d may be estimated at 0.0 to 13.9%, whereas retention after 100 d assumes values between 0.0 and 5.5% (Figure 3B).
In adolescents (15 y) and adults (20 y) respective trends detected after comparison of children with infants find their continuation. Both age groups are characterized by very similar retention curves, reflecting the high similarity of their lung morphometries. Therefore, 24 h rention may be numbered with 36.7 to 85.3%, whilst 25 d retention assumes values between 0.0 and 21.1%. Retention after 100 d indicating the slowest clearance phase is set between 0.0 and 9.4% (Figure 3C,D).
Clearance course for selected particle sizes
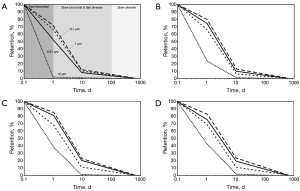
In order to get a more detailed insight into the clearance process of single bioparticles, time-dependent retention curves have been computed for four diameter classes (0.01, 0.1, 1.0 and 10 µm). As exhibited in the graphs of Figure 4, the plotted functions reproduce single clearance phases in an appropriate fashion. In infants retention of bioparticles with diameters of 0.1 and 1.0 µm clearly exceeds that of particles with diameters of 0.01 and 10 µm. According to the stochastic clearance model total clearance time of the size classes ranges from 65 to 580 d (Figure 4A). In the case of children particle-specific retention values are remarkably increased with respect to the values of infants, resulting in a respective prolongation of clearance duration. Therefore, total clearance time assumes values ranging from 128 to 650 d (Figure 4B). In adolescents and adults a further enhancement of particle-specific retention values may be observed, resulting in a renewed prolongation of the clearance process. In concrete terms, this phenomenon is marked by a duration assuming values between 156 and 680 d (Figure 4C,D).
Discussion and Conclusions
Experimental and theoretical clearance research conducted during the past decades (4-15) has come to the conclusion that the innate defense system of the human respiratory tract may vary with regard to its efficiency. Main factors having a noticeable effect on this variation are among other particle size and shape. Both properties, which play an essential role in association with bioaerosols and their particulate components (1,32), perform a direct influence on clearance, since geometry and size determine the slow clearance fractions in the bronchial and alveolar structures (4-12,19,20,21,24-27). In addition, an indirect effect on clearance is given by these two particle traits insofar as they significantly control the deposition behaviour of the inhaled substances and, as main consequence of that, the particulate fractions being accumulated in central and peripheral lung regions (1-12,33-36).
Previous studies provided the clear evidence that deposition of airborne particles inhaled by infants and children is characterized by valuable discrepancies with respect to the deposition of these particles in adolescents and adults (1,11,20,36). This age-dependent deposition is chiefly based upon two phenomena: First, infants’ and children’s lungs noticeably differ in size from adolescents’ and adults’ lungs, with most deposition mechanisms being more pronounced in small lung structures than in large ones. Second, young probands are distinguished by highly specific breathing behaviours with high breathing frequencies and small tidal volumes, whereas older subjects tend to continuously decrease the number of breath-cycles per minute and to permanently increase the tidal volume (1,11,20). The breathing behaviour in infants and children results in a preferential deposition of inspired particles in the upper and central airways. In contrast, adolescents and adults exhibit enhanced particulate penetration depths with preferred deposition sites of the inhaled substances being situated in the central and peripheral airways (1,11,19,20).
According to the results presented in this contribution clearance of biogenic particles has a higher efficiency in young probands and a lower one in older subjects. This circumstance seems to be surprising at first glance, because bronchial mucus velocities show a positive correlation with age (see above, 11). However, in this case reduced airways sizes in the lungs of infants and children play a rather decisive role. Although initial mucus velocities in infants assume values being 80% lower than those in adults, average lung sizes of 1-year-old probands amount to only 40% of those measured in adult subjects (1,11,20). Combination of these two observations and addition of the find, according to which particle deposition is significantly shifted to the upper airways in small lungs, leads to the result that clearance time in young subjects assumes values being 20% lower than those in adults.
Although particle filtering in the extrathoracic and upper bronchial airways as well as clearance efficiency show a negative correlation with proband’s age, long-term exposure to certain biogenic particles may induce diverse insufficiencies in the respiratory system (11,24,25). Among these possible diseases, allergic and inflammatory reactions may occur with highest frequencies, whereas more serious injuries causing a permanent modification of the epithelial structure and, as a consequence of that, the whole lung architecture possess a much lower probability. According to the results of the associated deposition study (1) and the modeling data presented here, biogenic particles of intermediate size (0.5–1.0 µm) may dispose of increased deposition fractions in the alveoli and, thus, bear an enhanced and age-independent hazardous potential. Theoretical studies, however, recommend the avoidance of long-term bioaerosol inhalation by either decreasing outdoor activities during periods of enhanced distribution of bioparticles in the atmosphere or keeping away from regions with increased bioaerosol concentration in the ambient air.
Based upon the theoretical data presented in part 1 and 2 of the bioaerosol study it may be concluded that biogenic particles play an essential role in occupational hygiene and, therefore, have to be subjected to further detailed studies in the future.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Sturm R.. Bioaerosols in the lungs of subjects with different ages-part 1: deposition modeling. Ann Transl Med 2016;4:211. [Crossref] [PubMed]
- Oberdörster G.. Lung clearance of inhaled insoluble and soluble particles. J Aerosol Med 1988;1:289-330. [Crossref]
- Wolff RK. Mucociliary Function. In: Parent RA. editor. Comparative Biology of the Normal Lung. New York: CRC Press, 1989:659-80.
- Sturm R, Hofmann W, Scheuch G, et al. Particle clearance in human bronchial airways: comparison of stochastic model predictions with experimental data. Ann Occup Hyg 2002;46:329-33. [PubMed]
- Sturm R, Hofmann W. Mechanistic interpretation of the slow bronchial clearance phase. Radiat Prot Dosimetry 2003;105:101-4. [Crossref] [PubMed]
- Hofmann W, Sturm R. Stochastic model of particle clearance in human bronchial airways. J Aerosol Med 2004;17:73-89. [Crossref] [PubMed]
- Sturm R, Hofmann W. Stochastic modeling predictions for the clearance of insoluble particles from the tracheobronchial tree of the human lung. Bull Math Biol 2007;69:395-415. [Crossref] [PubMed]
- Sturm R, Hofmann W.. A multi-compartment model for slow bronchial clearance of insoluble particles--extension of the ICRP human respiratory tract models. Radiat Prot Dosimetry 2006;118:384-94. [Crossref] [PubMed]
- Sturm R.. A computer model for the clearance of insoluble particles from the tracheobronchial tree of the human lung. Comput Biol Med 2007;37:680-90. [Crossref] [PubMed]
- Sturm R.. A computer model for the simulation of fiber-cell interaction in the alveolar region of the respiratory tract. Comput Biol Med 2011;41:565-73. [Crossref] [PubMed]
- Sturm R.. Theoretical models of carcinogenic particle deposition and clearance in children’s lungs. J Thorac Dis 2012;4:368-76. [PubMed]
- Sturm R.. Clearance of carbon nanotubes in the human respiratory tract-a theoretical approach. Ann Transl Med 2014;2:46. [PubMed]
- Stahlhofen W, Gebhart J, Rudolf G, et al. Measurement of lung clearance with pulses of radioactivity-labelled aerosols. J Aerosol Sci 1986;17:330-6. [Crossref]
- Stahlhofen W, Koebrich R, Rudolf G, et al. Short-term and long-term clearance of particles from the upper human respiratory tract as a function of particle size. J Aerosol Sci 1990;21:S407-10. [Crossref]
- Scheuch G, Stahlhofen W, Heyder J. An approach to deposition and clearance measurements in human airways. J Aerosol Med 1996;9:35-41. [Crossref] [PubMed]
- Lippmann M.. Effects of fiber characteristics on lung deposition, retention, and disease. Environ Health Perspect 1990;88:311-7. [Crossref] [PubMed]
- Gradoń L, Podgórski A. Kinetics of particle retention in the human respiratory tract. Ann Occup Hyg 1991;35:249. [PubMed]
- Sturm R.. Deposition and cellular interaction of cancer-inducing particles in the human respiratory tract: Theoretical approaches and experimental data. Thoracic Cancer 2010;4:141-52. [Crossref]
- Sturm R, Hofmann W.. A theoretical approach to the deposition and clearance of fibers with variable size in the human respiratory tract. J Hazard Mater 2009;170:210-8. [Crossref] [PubMed]
- International Commission on Radiological Protection (ICRP). Human Respiratory Tract Model for Radiological Protection, Publication 66. Oxford: Pergamon Press, 1994.
- Asgharian B, Hofmann W, Miller FJ. Mucociliary clearance of insoluble particles from the tracheobronchial airways of the human lung. J Aerosol Sci 2001;32:817-32. [Crossref]
- Lee PS, Gerrity TR, Hass FJ, et al. A model for tracheobronchial clearance of inhaled particles in man and a comparison with data. IEEE Trans Biomed Eng 1979;26:624-30. [Crossref] [PubMed]
- Yu CP, Hu JP, Yen BM, et al. Models for mucociliary particle clearance in lung airways.In: Lee SD, Schneider T, Grant LD, Verkerk PJ, editors. Aerosols: Research, risk assessment and control strategies. Chelsea: MI, Lewis, 1986:569-78.
- Sturm R.. Theoretical approach to the hit probability of lung-cancer-sensitive epithelial cells by mineral fibers with various aspect ratios. Thoracic Cancer 2010;3:116-25. [Crossref]
- Sturm R.. Age-dependence and intersubject variability of tracheobronchial particle clearance. Pneumon 2011;24:77-84.
- Sturm R.. Theoretical and experimental approaches to the deposition and clearance of ultrafine carcinogens in the human respiratory tract. Thoracic Cancer 2011;2:61-8. [Crossref]
- Sturm R.. An advanced stochastic model for mucociliary particle clearance in cystic fibrosis lungs. J Thorac Dis 2012;4:48-57. [PubMed]
- Sturm R.. A three-dimensional model of tracheobronchial particle distribution during mucociliary clearance in the human respiratory tract. Z Med Phys 2013;23:111-9. [Crossref] [PubMed]
- Sturm R.. Theoretical models for the simulation of particle deposition and tracheobronchial clearance in lungs of patients with chronic bronchitis. Ann Transl Med 2013;1:3. [PubMed]
- Sturm R.. Modeling the delay of mucous flow at the carinal ridges of the human tracheobronchial tree. Comp Math Biol 2014;3:6.
- Sturm R.. An advanced mathematical model of slow bronchial clearance in the human respiratory tract. Comp Math Biol 2016;5:2.
- Sturm R.. Modeling the deposition of bioaerosols with variable size and shape in the human respiratory tract - A review. J Adv Res 2012;3:295-304. [Crossref]
- Sturm R, Hofmann W.. A computer program for the simulation of fiber deposition in the human respiratory tract. Comput Biol Med 2006;36:1252-67. [Crossref] [PubMed]
- Sturm R.. Theoretical models for dynamic shape factors and lung deposition of small particle aggregates originating from combustion processes. Z Med Phys 2010;20:226-34. [Crossref] [PubMed]
- Sturm R.. Nanotubes in the respiratory tract - Deposition modeling. Z Med Phys 2015;25:135-45. [Crossref] [PubMed]
- Sturm R.. A stochastic model of carbon nanotube deposition in the airways and alveoli of the human respiratory tract. Inhal Toxicol 2016;28:49-60. [Crossref] [PubMed]


