Primary psoas sarcoma causing malignant psoas syndrome: favourable response to radiotherapy
Introduction
A paper published by Stevens et al. (1) discussed the malignant psoas syndrome (MPS), a cluster of symptoms related to malignant involvement of the psoas muscle and infiltration of the lumbosacral nerve plexus with resultant neurological signs and symptoms. We wish to bring attention to the role that radiotherapy may play in the management of this syndrome.
Case presentation
Mrs X, a 68-year-old woman, was referred to our department for consideration of radiotherapy to a left psoas muscle mass.
Mrs X developed low back pain about 8 months prior to presentation. This pain radiated down her left leg to the anteromedial thigh. At the suggestion of her chiropractor, she underwent imaging (including CT, MRI and PET) that demonstrated invasion of the left psoas muscle by a probable malignant process and destruction of the left lateral body of the L4 vertebra. Biopsy of the lesion suggested a low grade sarcoma but further classification was not possible, despite additional expert review. Mrs X was reviewed by an orthopaedic and oncological surgeon and referred to radiation oncology for consideration of neoadjuvant radiotherapy.
Mrs X’s past medical history was significant for ischaemic heart disease and type 2 diabetes mellitus complicated by peripheral sensory neuropathy. There was no evidence of retinopathy or nephropathy. She had previously required angioplasty and stenting for coronary artery disease. Her performance status was ECOG 1.
Examination findings at the time of first presentation demonstrated midline bony tenderness overlying the 4th lumbar vertebra, but there was no evidence of neurological impairment. The left leg was maintained in a flexed position and the reverse straight leg raise test (psoas stretch test) was positive.
Mrs X received a total radiotherapy dose of 50.4 Gray in 28 fractions (1.8 Gray/fraction) over 6 weeks with a 3D conformal technique. Radiation was delivered to the abdominal and most of the pelvic portion of the psoas muscle and tumour mass. A coronal beams-eye view of the anterior treatment field is shown in Figure 1.
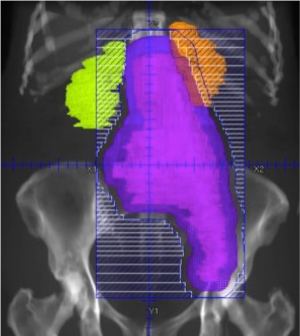
Follow-up pre-operative MRI of the lesion demonstrated a small decrease in its size. Her pain improved significantly during this time without any increase in analgesics or co-analgesics, improving mobility and subjective quality of life.
Five weeks later, Mrs X underwent a subtotal L4 vertebrectomy with reconstruction and posterior fixation, and the left psoas muscle was resected from the level of L3 to L5. The left obturator and genitofemoral nerves were sacrificed, as was the L4 spinal nerve and the L4 root of the femoral nerve.
The histopathology report concluded that the appearance was consistent with “a low grade but locally aggressive sarcoma of uncertain classification” with morphologic appearance suggestive of solitary fibrous tumour. Such intermediate grade soft tissue tumours rarely metastasise. These results appeared somewhat incongruous with the partial destruction of the L4 lumbar vertebra by the tumour and subsequently the surgical specimen was sent for expert opinion to a pathologist in Italy who confirmed the diagnosis of solitary fibrous tumour.
Post-operatively, the loss of the L4 root of the femoral nerve led to significant weakness of the knee and the patient required a knee brace for mobilisation. Loss of the left obturator nerve resulted in significant ipsilateral hip adductor weakness. Significant paresthesia of the thigh and medial lower leg also persisted. She remained disease free 3 years post-surgery.
Discussion
MPS, as first described by Stevens et al. (2) in 1990, is characterised by “clinical evidence of proximal lumbosacral plexopathy, painful fixed flexion of the ipsilateral hip” and confirmation of tumour infiltration into the proximal psoas muscle by either imaging or histopathology. Symptoms may also include pain or weakness in the distribution of the peripheral nerves originating from the lumbar plexus. To date, around 30 cases have been reported in the literature (Table 1), with cases reported since the 2010 update by Stevens et al.
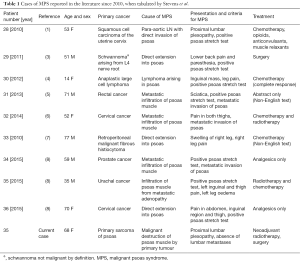
Full table
The psoas major has muscular attachments arising from the transverse processes of the 12th thoracic vertebra to the 5th lumbar vertebra which contribute to the deep component of the muscle. The superficial component originates from the lateral aspect of the T12-L4 vertebral bodies. As such, the nerve roots emerge through the transverse foramina between the superficial and deep parts of the psoas. The psoas major extends inferolaterally from its origin where it joins the iliacus to form the iliopsoas which ultimately inserts into the lesser trochanter (9). The psoas muscles are innervated by the lumbar plexus.
The lumbar plexus is formed from the ventral rami of L1 to L4 within the posterior one third of the psoas major muscle, anterior to the transverse processes of the lumbar vertebrae. There are six major branches extending beyond the lumbar plexus—the iliohypogastric, ilioinguinal, genitofemoral, lateral femoral cutaneous, femoral and obturator nerves (9). These nerves provide motor supply to muscles of hip flexion, hip adduction and knee extension. Sensation supplied by these nerves includes the skin of the inferior abdomen, the external genitalia, the medial, anterior and lateral surfaces of the thigh and the medial surface of the lower leg to the first metatarsal in the foot (10). Variable presentations in terms of paresthesias and weakness are understandably linked to the level at which pathology arises and its degree of infiltration of the plexus. Positive reverse straight-leg raise test or femoral stretch test is suggestive of L2-4 nerve root involvement and results in ipsilateral anterior thigh or medial leg pain.
Broadly speaking pain is characterised as being either nociceptive or neuropathic in origin, however many patients with MPS present with features of both. Nociceptive pain arises from mechanical, chemical or thermal irritation of peripheral sensory nerve endings within skin and deep tissues. It can be further divided into somatic (sharp, localised, incident) or visceral (dull, cramping, colicky) nociceptive pain according to site of origin. Neuropathic pain, in contrast, results from direct injury to nerves or dysfunction of the somatosensory system in response to uncontrolled pain (sensitisation). Neuropathic pain may occur in concert with changes in nerve function such as motor deficits. Sensitisation is the reduction of pain threshold in injured tissues, compounded by injury to the nerves supplying that tissue.
In MPS both nociceptive and neuropathic pain pathways are activated and this makes the management of the pain associated with this pathology particularly difficult to manage. As such, it may be necessary to use multiple analgesics and co-analgesic agents to control symptoms.
Retroperitoneal sarcoma has been reviewed by Porter et al. (11) and consists of a diverse group of rare malignancies that originate from mesenchymal cells. Solitary fibrous tumours, as reported here, are typically intermediately differentiated soft tissue tumours of mesenchymal origin which, whilst rarely metastasizing, do have malignant potential (12). As such, they do not strictly meet criteria for being fully benign but this is indeed a spectrum. Patients may present with a painless abdominal mass, or alternatively from symptoms due to mass effect or local invasion. Surgery, which plays a major role in the curative treatment of most sarcomas, is difficult in this region, as clear margins are elusive. Radiotherapy is administered in about 26% of cases (11), usually post-operatively. It has been suggested that pre-operative radiotherapy for treatment of retroperitoneal sarcoma may be preferable as it is likely to “provide the highest chance of safe and successful delivery of multimodal therapy” which can be hindered by post-surgical changes and the challenging anatomy associated with these tumours (13).
Sarcomas arising in the psoas muscle are relatively rare (about 1% of all soft tissue sarcomas) (11). Common presenting symptoms include pain along the femoral nerve and a mass. Most recently, a case similar to Mrs X was described by Hammouche et al. (14) of an iliopsoas sarcoma causing referred pain to the inguinal and thigh areas.
Stevens et al. (1) discusses numerous methods of treating MPS, including standard measures such as surgery and pharmacotherapy. More novel measures include intrathecal analgesia, local anaesthesia of the psoas sheath and cordotomy, all of which are invasive procedures.
In their 2004 article, Behranwala et al. (15) explored the natural history of malignant tumours of the iliopsoas compartment. Twelve of the 19 patients presented in the series underwent neoadjuvant, adjuvant or palliative radiotherapy, however the results of this treatment were not detailed. The authors suggest that the poor prognosis of sarcoma at this site is related to delayed diagnosis from non-specific symptoms and an unfavourable anatomic location relative to vital structures.
To the authors knowledge there have been no randomised controlled trials examining the role of radiotherapy in the neoadjuvant or adjuvant treatment of primary retroperitoneal sarcoma. As such, the employment of radiotherapy in this context is reliant upon observational data. Le Péchoux et al. (16) reported the results of their analysis of 110 patients with primary retroperitoneal sarcoma who underwent front-line aggressive surgery with or without adjuvant radiotherapy (to a median dose of 50 Gy) between 1994 and 2008. Whilst the results of this analysis failed to detect a statistically significant difference in local failure rates or overall survival between the groups, relapse free survival was 47% in the surgery alone arm versus 60% with the addition of post-operative radiation (P=0.02) at five years. A retrospective review by Alford et al. (17) also supports the use of radiation therapy in combination with surgery for the treatment of retroperitoneal sarcoma. The authors favoured a neoadjuvant approach (45–50 Gy at 1.8 Gy per fraction) with the aim of improving resection margins and minimising toxicity. The outcomes of twenty four patients who completed preoperative radiotherapy were detailed with 53.7% of patients alive at five years and 22% free of local recurrence. Eighteen patients proceeded to resection following radiation with clear margins achieved in all cases.
The primary aim of management of retroperitoneal sarcoma is to achieve complete resection with robust margins free of tumour involvement (18). Local failure has been closely linked to survival in numerous studies (11,18) and it follows that if radiotherapy improves local control then benefits in overall survival may also be realised with time. In the setting of MPS, if symptoms are relieved with resection and radiotherapy improves failure rates, then symptoms should be less likely to recur in the absence of recurrent disease. Improved conformality of treatment with use of intensity-modulated radiation therapy (IMRT) and intra-operative radiation therapy to boost dose to the tumour bed are promising areas of development which are likely to influence clinical outcomes.
Toxicity from radiotherapy to the abdomen/retroperitoneum includes early effects such as fatigue, nausea, diarrhoea and skin erythema. These effects are easily managed with modern pharmaceuticals. Late effects, which occur at least 3 months following radiation treatment, are uncommon but may include small bowel damage (obstruction, fistulae) and cosmetic skin changes. Radiation myelopathy is a rare complication (<0.2% patients) at doses to the spinal cord of less than 50 Gy (19). It is also important to note that the position of the cauda equina at the approximate level of the L2 lumbar vertebra, beyond the conus medullaris, further reduces the risk of spinal cord myelopathy secondary to radiotherapy targeted in this region. Generally, for patients with incurable malignancy, the likelihood of developing complications in their remaining lifespan is very low.
Radiotherapy can improve pain caused by MPS and should be considered as a therapeutic option. It is non-invasive and acute side effects are easily managed with modern pharmaceuticals. Radiotherapy works locally and allows patients to reduce their analgesic requirements. Patients are likely to gain greatest benefit from a multidisciplinary approach to the management of their disease within a dedicated cancer centre.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: The patient has given her consent for us to publish this de-identified image of her radiotherapy treatment.
References
- Stevens MJ, Atkinson C, Broadbent AM. The malignant psoas syndrome revisited: case report, mechanisms, and current therapeutic options. J Palliat Med 2010;13:211-6. [Crossref] [PubMed]
- Stevens MJ, Gonet YM. Malignant psoas syndrome: recognition of an oncologic entity. Australas Radiol 1990;34:150-4. [Crossref] [PubMed]
- Shimoda Y, Morimoto D, Isu T, et al. Schwannoma developing in the psoas major muscle: a case report. No Shinkei Geka 2011;39:51-7. [PubMed]
- Kounami S, Shibuta K, Yoshiyama M, et al. Primary anaplastic large cell lymphoma of the psoas muscle: a case report and literature review. Acta Haematol 2012;127:186-8. [Crossref] [PubMed]
- Komatsu S, Iseki M, Morita Y, et al. A case of malignant psoas syndrome diagnosed while treating sciatica. Masui 2013;62:863-6. [PubMed]
- Basu S, Mahajan A. Psoas muscle metastasis from cervical carcinoma: Correlation and comparison of diagnostic features on FDG-PET/CT and diffusion-weighted MRI. World J Radiol 2014;6:125-9. [Crossref] [PubMed]
- Yamamoto Y, Goto M, Okamoto T, et al. Chemotherapy-naïve advanced malignant fibrohistiocytoma presenting IVC syndrome case report. Gan To Kagaku Ryoho 2010;37:355-7. [PubMed]
- Takase N, Ikegaki J, Nishimura H, et al. Methadone for Patients with Malignant Psoas Syndrome: Case Series of Three Patients. J Palliat Med 2015;18:645-52. [Crossref] [PubMed]
- Mirilas P, Skandalakis JE. Surgical anatomy of the retroperitoneal spaces, Part IV: retroperitoneal nerves. Am Surg 2010;76:253-62. [PubMed]
- Gray's Anatomy, 40e. Standring S. editor. Elsevier, Spain, 2008.
- Porter GA, Baxter NN, Pisters PW. Retroperitoneal sarcoma: a population-based analysis of epidemiology, surgery, and radiotherapy. Cancer 2006;106:1610-6. [Crossref] [PubMed]
- Fletcher DM, Unni KK, Mertens F. editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. IARC Press, Lyon, 2002.
- Mohindra P, Neuman HB, Kozak KR. The role of radiation in retroperitoneal sarcomas. Curr Treat Options Oncol 2013;14:425-41. [Crossref] [PubMed]
- Hammouche S, Mohammed I, Maheswaran T, et al. A rare clinical presentation of a malignant fibrous histiocytoma within the iliopsoas muscle. Ann R Coll Surg Engl 2010;92:W18-20. [Crossref] [PubMed]
- Behranwala KA, A'Hern R, Thomas JM. Primary malignant tumors of the iliopsoas compartment. J Surg Oncol 2004;86:78-83. [Crossref] [PubMed]
- Le Péchoux C, Musat E, Baey C, et al. Should adjuvant radiotherapy be administered in addition to front-line aggressive surgery (FAS) in patients with primary retroperitoneal sarcoma? Ann Oncol 2013;24:832-7. [Crossref] [PubMed]
- Alford S, Choong P, Chander S, et al. Outcomes of preoperative radiotherapy and resection of retroperitoneal sarcoma. ANZ J Surg 2013;83:336-41. [Crossref] [PubMed]
- Shiraev T, Pasricha SS, Choong P, et al. Retroperitoneal sarcomas: a review of disease spectrum, radiological features, characterisation and management. J Med Imaging Radiat Oncol 2013;57:687-700. [Crossref] [PubMed]
- Kirkpatrick JP, van der Kogel AJ, Schultheiss TE. Radiation dose-volume effects in the spinal cord. Int J Radiat Oncol Biol Phys 2010;76:S42-9. [Crossref] [PubMed]


