Considerations for analgosedation and antithrombotic management during extracorporeal life support
Introduction
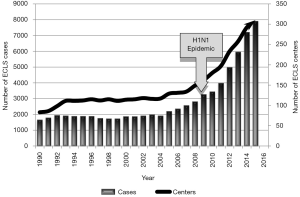
The application of extracorporeal life support (ECLS) has seen tremendous growth and advancement in recent years. Since the year 2000, the Extracorporeal Life Support Organization (ELSO) reports an ELCS case growth rate of nearly approximately 380% in the total number of neonates, pediatrics, and adults that have undergone ECLS (Figure 1). An aggregate ECLS survival rate is reported as 70%, with a 58% survival rate to discharge or transfer after ECLS—an improving number despite patient populations that are severely critically ill (1). Much advancement has enabled this growth including technological advancements in the devices and equipment incorporating, an improved understanding of blood rheology for better biocompatibility and overall improved patient selection and management.
Despite this growth there is an increasing awareness that some supportive therapies need greater focus to characterize management considerations during ECLS—one of these areas is pharmacotherapy. When investigating the evidence surrounding the pharmacotherapy during ECLS, one would hope to see similar expansive growth mimicking that demonstrated in Figure 1. However, when using broad PubMed MESH terms [“Pharmacokinetics” (Mesh) OR “Pharmacologic Actions” (Mesh) OR “pharmacology” (Subheading) OR “Pharmacology” (Mesh)] AND [“Extracorporeal Membrane Oxygenation” (Mesh)] the query demonstrates approximately 250% growth since 2000 with 2,487 total publications since 1990 (Figure 2). Despite this growth, many of the publications are case reports or case series or are limited in application across ECLS centers. Nonetheless, the data does indicate that additional considerations may need to be accounted for during ECLS superimposed upon those factors already present in this critically ill patient population. To further complicate matters, much of the pharmacotherapy data becomes difficult to interpret in light of the recent technological advancements in this arena. The evolution of the oxygenator and tubing used at minimum contribute to difficulty in extrapolating older data to today’s equipment. When multiplying the various combinations of ECLS circuit setups and proprietary equipment that may influence drug-circuit interactions across at least 300 international ECLS centers, it becomes very difficult to deduce objective pharmacotherapeutic guidance for this population.
Principles of pharmacotherapy in ECLS
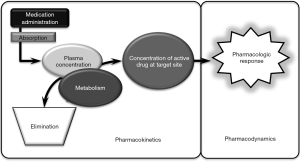
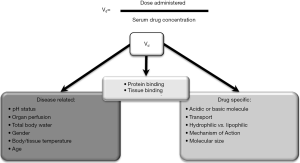
To put it into perspective, pharmacotherapy in the critically ill presents the challenge of weighing the risk of an adverse drug event or toxicity specific to the therapeutic index of a medication against the urgent need for a clinical effect in patients with dynamically changing end-organ function. In the critically ill, clinicians must consider the multimodal medication interactions that may present including drug-patient, drug-disease and drug-drug interactions to achieve the desired pharmacodynamic response (Figure 3). Due to injury or a decline in end-organ function, it is common for the critically ill to exhibit decreases in metabolism, elimination and ultimately decreased clearance. Volume of distribution is perhaps the more underappreciated pharmacokinetic change as it is influenced by a number of factors in the critically ill (Figure 4). Beyond routine care and therapeutic drug monitoring, the clinician is faced with the following decisions surrounding pharmacotherapy during ECLS:
- Use physiologic response to guide decisions regardless of potential drug-circuit interactions;
- Use laboratory based therapeutic drug monitoring to guide patient–specific decisions on medication levels regardless of suspected drug-circuit interactions;
- Use established evidence to guide management based on supporting data from drug-circuit interactions in ECLS or cardiopulmonary bypass literature;
- Apply pharmacotherapeutic principles using drug properties to identify potential drug-circuit interactions and seek alternative therapies, adjust dosing strategy and/or escalate monitoring.
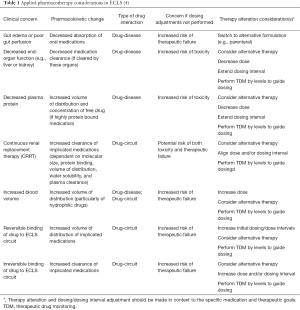
In ECLS patients there are additional considerations that must be accounted for to navigate the risk of toxicity versus the risk of therapeutic failure (Table 1). Different physiochemical properties of medications such as lipophilicity, polarity, and protein binding dictate the overall absorption, distribution, metabolism and excretion of a given agent. In ECLS, the increased volume of distribution and increased clearance seem to be best demonstrated by agents that have high lipophilicity, high protein binding, or high polarity molecules at a physiologic pH of blood (5-8). Clinicians involved in the management of ECLS should have a general understanding of the physiochemical properties that may play a role in their pharmacotherapy decision-making. Lipophilicity is best represented by the Log P value or octanol-water coefficient of a medication or molecule. Log P is derived using the logarithm of the comparative ratio the concentration of the solute with octanol and water. Larger values indicate increased lipophilicity, while lower values (particularly negative values) indicate greater hydrophilicity. Polarity and protein-binding are interdependent and help determine not only the extent of protein binding but also the tendency of what proteins may bind to certain medications. As such, Shekar and colleagues have demonstrated that while comparing medications with similar log P values, protein binding was the driving determinant of the ELCS circuit interaction in an ex-vivo model (8,9). Reference values of these physiochemical attributes for many agents are easily retrievable via pharmacy reference texts, or medicinal chemistry resources such as www.chemicalize.org or www.drugbank.ca.
The predominate sources of these drug-circuit interactions among current ECLS components include the polyvinyl chloride (PVC) tubing and polymethylpentene fibers in the most commonly used modern ECLS oxygenators (2,10-12). The use or application of data from ECLS models containing silicone-based tubing or silicone membrane oxygenators further comes with limitations, as silicone-based components have shown to potentially have a greater likelihood of drug-circuit interactions in comparison to PVC and Teflon (13). Although hypothesized, there is little characterization of a potential adsorption saturation ceiling points that could exist among medications to these different artificial surfaces. Although active investigations are ongoing, the characterization and adsorptive capacity beyond 24 hours of ECLS support remains untested to date, leaving clinicians without evidence to guide management (14). While most if not all medications used during the management of patients undergoing ECLS, analgosedation and antithrombotic management are common challenges among the whole ECLS population.
Analgosedation in ECLS: therapeutic management considerations
Management of analgosedation has taken a paradigm shift in recent years. It used to be viewed as humane practice to keep mechanically ventilated patients sedated with continuous infusion of sedatives and analgesia. While many other ICU factors beyond ECLS including frequent lab draws, finger sticks, and monitor alarms can cause physiological and psychological stress on the patient, the practice around analgosedation management requires diligent management as some of the agents and clinical targets have been associated delirium manifestation. Recent data supports the liberation of patients from analgosedation has been associated with fewer ICU days, less post-traumatic stress disorder, and other benefits, however in the ECLS population, this is fine a balance. While light sedation may be appropriate for many ECLS patients, it may not be appropriate for all. Patients that have undergone centralized cannulation may require a deeper sedation to prevent unwanted manipulation of the cannulas due to patient movement. Additionally, patients who present with acute respiratory distress syndrome (ARDS) who require a deeper level of sedation to prevent barotrauma or if continuous neuromuscular blockade is needed.
The Society of Critical Care Medicine (SCCM) recommends analgesia first sedation with light sedation goals for most critically ill patients (15). Adequate sedation may not be achieved with analgesics alone and the addition of a sedative may be required. The management of pain and agitation for a patient on ECLS should be similar to other critically ill patients, however, the patient’s clinical status and pharmacokinetic alterations due to the ECLS circuit should be considered to optimize analgesia and sedation. Regardless of the specific goal for analgesia and sedation, SCCM recommends frequent monitoring with validated scoring tools to ensure the patient stays within their goal level of pain and sedation.
Analgesia
Intravenous opioids have been mainstay of treatment for non-neuropathic pain in the intensive care unit (ICU). Historically, morphine has been regarded as the preferred opioid for analgesic therapy in the ICU (16). It is appealing for patients on ECLS support because it is fairly hydrophilic which may prevent it from being sequestered into the ECLS circuit. In closed ECLS circuits, morphine concentrations have been shown to be reduced by only 10–20% at 24 hours (8,17,18). Despite this pharmacokinetic advantage, morphine has some undesirable characteristics, which has limited its use in the ECLS population. Morphine may provoke histamine release, resulting in vasodilation, which could contribute to hypotension and hemodynamic instability (19). In addition, it’s half-life of 4–5 hours are among the longest for intravenous opioids. Lastly, morphine’s metabolite morphine-3-glucuronide accumulates in patients with renal dysfunction and decreases the patient’s seizure threshold (20). Due to these disadvantages, many centers use alternative agents.
Fentanyl, a synthetic opioid, is commonly used in the ICU because it has a rapid onset of action, short half-life of 1–3 hours, and minimal effect on hemodynamics, making it easily titratable with limited adverse effects (21). The high lipophilicity of fentanyl (log P of 3.9) contributes to its ease in passage through the blood-brain barrier to exert its clinical effect (22). However, during ECLS, its lipophilicity is thought to contribute to significant adsorption to the tubing of the of the circuit. Fentanyl concentrations have been shown to be reduced by 80–100% after 24 hours in a closed ECLS circuit (9,18,21). Due to significant amounts of drug being sequestered into the ECLS circuit, higher doses may be required to achieve the patient’s pain and sedation goals than seen in non-ECLS patients.
The ideal agent for analgesia in ECLS patients may be hydromorphone. Hydromorphone is a semi-synthetic opioid with little to no effect on hemodynamics and a rapid onset of action, similar to fentanyl, but a longer half-life of 2–3 hours, which allows it to be used as a bolus or continuous infusion (22). Hydromorphone may accumulate in renal and hepatic impairment; however it does not have any harmful or active metabolites, as morphine does. Hydromorphone is more hydrophilic then fentanyl, which may prevent it from binding the ECLS circuit (22). Due to the potential of having less drug bind to the ECLS circuit, patients may achieve their pain and sedation goals more readily with hydromorphone compared to fentanyl and without the hemodynamic disadvantages of morphine.
Sedation management
When analgesia alone cannot achieve adequate sedation or the clinical situation calls for a deeper level of sedation, a sedative agent may be warranted. Benzodiazepines have traditionally been the sedative of choice in the critically ill (16). Benzodiazepines have anxiolytic and amnestic properties by activating the This should be written “gamma-aminobutyric acid” or use the greek symbol for gamma receptors (GABA) (19). Two commonly used parenteral benzodiazepines used in the ICU are lorazepam and midazolam. Midazolam is usually the agent of choice for continuous sedation as lorazepam is not water-soluble and requires propylene glycol as a diluent (23). Prolonged infusions of lorazepam (>24 hours) and/or higher doses (>10 mg/hr) have been shown to cause accumulation of propylene glycol, which is associated with hyperosmolar metabolic acidosis, lactic acidosis, acute tubular necrosis, seizures, cardiac arrhythmias, and hypotension (23). Due to this, lorazepam is usually avoided for situations in which continuous sedation is needed. Midazolam is water soluble, with an onset of action between 2–5 minutes, and a shorter half-life than lorazepam (3–12 vs. 10–20 hrs), all of which make it more desirable for continuous sedation in ECLS patients (19). As is common with many sedatives, both of these parenteral agents are quite lipophilic—lorazepam has a log P of 3.5, while midazolam is even more lipophilic with a log P of 3.9 (6,21). Since midazolam is very lipophilic it has been shown to be sequestered within the ECLS circuit. In a closed ECLS circuit, concentrations of midazolam were reduced by over 90% (24). Even though midazolam is sequestered within the ECLS circuit it is still preferred over lorazepam based on its other pharmacokinetic properties. When midazolam is utilized the clinician should be aware of drug loss within the circuit and titrate doses to the desired response.
Benzodiazepines have been the cornerstone medications used for sedation, however, data among the aggregated critically ill population continues to emerge that the use of benzodiazepines is associated with increased rates of delirium, time on mechanical ventilation, and ICU length of stay compared to propofol and dexmedetomidine (25-27). Based on this evidence, there has been a shift to non-benzodiazepine based sedation regimens. Propofol is an attractive option because of its quick onset of action and short half-life, however propofol has among the highest lipophilicities in the agents for sedation with a log P of 4—so lipophilic that it requires a lipid emulsion vehicle (6,19). When studied in an ex-vivo model using closed ECLS circuits, 95–100% of the propofol infusion was sequestered within the ECLS circuit (7,24). Propofol’s lipophilicity along with its hypotensive effects limit its use for continuous sedation in the ECLS population.
Dexmedetomidine is a selective α2 agonist with approximately 8 times more specificity for the α2 receptor than clonidine. Dexmedetomidine has a short half-life of 30 minutes with limited effect on respiratory function, making it an appealing sedation option (28). The α2 agonist mechanism, allows dexmedetomidine the added benefit of some analgesic activity as well as sedation. However, due to activation of presynaptic α2 receptors, dexmedetomidine can inhibit the release of norepinephrine and epinephrine leading to hypotension and bradycardia. Since dexmedetomidine can decrease a patient’s sympathetic tone, it may not be the ideal agent for patients requiring hemodynamic support with inotropes and/or vasopressors. Specifically, patients in cardiogenic shock requiring VA ECLS may benefit from a different agent. In addition, dexmedetomidine has been shown to bind to ECLS circuits, with up to 90% reduction in concentrations after 24 hours in a closed ECLS circuit (29). Due to its side effect profile and amount of drug lost in the ECLS circuit, dexmedetomidine should be used cautiously with this patient population.
Analgesia and sedation are a crucial part of caring for a patient on ECLS. The physiological and psychological stress patients undergo while being on mechanical circulatory support can have both short- and long-term consequences (30). Proper analgesia and sedation can help blunt this stress response, decrease metabolic demand, and provide patient comfort (31). Data regarding analgesia and sedation for patients on ECLS is limited and it is important to understand the majority of the pharmacokinetic data is derived from short term ex vivo experiments. When caring for a patient on ECLS it is crucial to consider the pharmacokinetic and dynamic properties of the drug, type and duration of ECLS, and patient factors that influence drug dosing in order to prevent harm or therapeutic failure.
Anticoagulation, thrombosis, hemostasis and monitoring in ECLS
Blood is constantly exposed to the foreign material of the ECLS circuit which leads to stimulation of the coagulation cascade and development of inflammation. The hypercoagulable state ECLS has long been well reviewed and culminating into the 2014 ELSO Anticoagulation Guideline help to aggregate the need for antithrombotic agents and diligent monitoring (32,33). However, Kruger et al, recently published a case series on patient requiring venovenous ECLS and utilizing deep vein thrombosis prophylactic subcutaneous low molecular weight heparin (LMWH) only (34). They evaluated 61 patients who received prophylactic anticoagulation and observed no fatal thrombotic events and less bleeding than reported in the current registry (34). Despite this evidence along with case reports, one must carefully weigh the risk of bleeding with the risk of clotting when evaluating for the need and timing of anticoagulation in ECLS patients (32,35). Among the 300 or more registered ECLS centers internationally, while most have center-specific common practices around antithrombotic management, the practices as a whole are widely non-standardized. Aside from clinician experience and preference, other contributing factors include variability in agent and monitoring assay access, intracenter and intercenter variability of the assay results, and overall clinical and operational management that coincides.
Coagulation monitoring
There are a number of tests currently available to assess the coagulation status of a patient. The optimal monitoring tests in ECLS have not been established. All the currently available tests come with their limitations which need to be taken into account when standardizing an approach to anticoagulation monitoring at the ECLS center. Sampling with multiple tests several times a day will most likely lead to disparate results and management decisions (32,36).
ACT
Activated clotting time (ACT) is a functional, whole blood, point of care test where blood is mixed with an activator such as kaolin. It provides insight on the coagulation response of the whole blood and does not correlate with activated partial thromboplastin time (aPTT), antifactor Xa assay (anti-Xa), or heparin levels (37). However, it does offer a quick, crude result with many centers targeting an ACT of 180-220 secs in ECLS (37). However, at many centers the ACT has fallen out of favor in recent years for monitoring outside of a procedural area because it is easily influenced by many factors including hypothermia, platelet dysfunction, hemodilution, etc., and results vary amongst devices (32,38). Commonly the devices offer cartridges specific to high- versus low-range ACT sensitivity. However, further limitations exist with results at the high end and low end of the ranges of the respective cartridges that may result in variability in management if the wrong sampling cartridge is used.
Anti-Xa
Anti-Xa is utilized by many centers in the management of unfractionated heparin and LMWH with a target therapeutic range of 0.3–0.7 IU/mL (37). Anti-Xa is a chromogenic assay that approximates the effect of heparin on hemostasis. There are two types of anti-Xa tests available that differ in the presence or absence of exogenous antithrombin (37). Many centers have moved toward testing without the addition of exogenous antithrombin as to give a true representation of the heparin effect on the patient’s coagulation status especially in those with low antithrombin. However, it is important to verify the tests performed at your laboratory. One of the clinical limitations of the anti-Xa assay is the potential influence other contributors to hemostasis including liver disease, factor deficiencies, hemolysis, disseminated intravascular coagulation, and vitamin K deficiency (39-41). However, anti-Xa monitoring is recommended in patients who have a baseline elevated aPTT from lupus anticoagulant (42).
aPTT
The activated partial thromboplastin time (aPTT) is a test that measures the intrinsic and common coagulation pathways and has been used for years to monitor systemic heparin (37,39). The aPTT is performed by surface activator and phospholipid to citrated plasma which results in a clotting time (measure in seconds) (37,39,43). Aside from influences caused by sampling techniques and confounding patient attributes (e.g., antiphospholipid antibodies), a large contributor to variability is the wide range in sensitivity among the reagents used for this assay. This was first described by Brill-Edwards and collegues in 1993, leading many labs to develop institution specific correlation curves for the heparin therapeutic ranges that correspond to a specified level of heparin (44). One of the limitations of aPTT testing is the variability in therapeutic ranges because different reagents utilized. Laboratories should establish new therapeutic goals as the reagent changes (39). The heparin therapeutic range in most centers corresponds to an anti-Xa level of 0.3–0.7 IU/mL (37). This heparin therapeutic range of 0.2 to 0.4 unit/mL was established originally using protamine titration and eventually translated to anti-Xa levels (45). Context would also note that this therapeutic target range was derived from venous thrombosis model in New Zealand white rabbits yet has been considerably extrapolated to the practices in anticoagulation seen today (45).
Antithrombin
Monitoring of antithrombin (AT) may be useful in those patients who are requiring high doses of heparin with suspected heparin resistance. There is currently no standard recommendation of antithrombin monitoring in those patients receiving a heparin infusion requiring ECLS. However, Bembea et al., surveyed 187 ELSO centers regarding anticoagulation monitoring. In this survey, 82% of respondents (65% response rate) reported antithrombin testing with 78% reporting at least daily of antithrombin (46). On the other hand, many centers do not currently measure antithrombin routinely and laboratory assays vary widely among institutions (32).
Thromboelastography (TEG) or rotational thromboelastography (ROTEM)
TEG is a viscoelastic test for hemostasis that has been used for years to evaluate global clot formation and kinetics both in the surgery and trauma population, while ROTEM is updated technology (32,37,47). Both TEG and ROTEM are point of care tests that help identify specific deficits hemostatic factors to guide transfusion, anticoagulation, and hemostatic agent usage (32,37,47). Although many centers are currently utilizing TEG/ROTEM monitoring, more studies are needed to clarify the role and usage of these tests in the ECLS population (32,37).
Antithrombotic agents
Heparin
Heparin continues to be the anticoagulant of choice for ECLS given its familiarity, reversibility, rapid onset, and lower cost compared to the parenteral direct thrombin inhibitors (DTI) (32,37). Heparin is a glycosaminoglycan that binds to antithrombin via a pentasaccharide sequence resulting in a conformational change. This conformational change allows the heparin-antithrombin complex to accelerate its indirect anticoagulant effect of inhibiting thrombin and factor X (32,48).
When initiating ECLS, most centers will bolus a patient with heparin 50–100 units/kg then start a continuous infusion once hemostasis has been achieved (32). However, there are some limitations with heparin including the need for antithrombin to exhibit its anticoagulant effect, heparin is a platelet activator, risk of development of heparin induced thrombocytopenia (HIT), lack of a linear dose response, in addition to lacking effect on clot bound thrombin. This has led some institutions to use alternative agents including direct thrombin inhibitors (DTIs) (49).
Antithrombin
AT is required to be able to exert the anticoagulant effects of heparin. It is postulated that patients may require AT supplementation while on ECLS to prevent or treat higher dose requirements or ineffectiveness of heparin. Some centers routinely monitor AT and treatment to a specified goal whereas others only replace AT when heparin resistance is suspected. Ryerson et al. evaluated the use of AT in 36 children and found the administration of AT resulted in decreased heparin dose requirements (50). On the other hand, Byrnes et al., compared children who received AT versus those who did not during ECLS and their group found no change in heparin responsiveness (51). The off-label use of antithrombin concentrate during ECLS remains controversial (32). Current data is limited to the pediatric population. Infants inherently have lower AT activity and levels so it is difficult to extrapolate this data to the adult population (32).
Direct thrombin inhibitors (DTIs)
Data have been emerging with DTIs in the ECLS population. DTIs are a particularly attractive alternative to heparin given lack of HIT, relatively short half-life, the ability to bind to clot bound thrombin, and more predictable response resulting in less dosing changes. On the other hand, DTI currently lack an available reversal agent. While there are consideration surrounding each agent overall, dosing and monitoring of the respective DTI is generally based upon institutional practice, coagulation monitoring, comorbidities, and goals of anticoagulation.
Argatroban is approved in the United States for use in patients with suspected or confirmed HIT. It has a half-life of approximately 40–50 minutes and 65% is eliminated via the hepatobiliary and 22% is cleared renally (52). This can lead to challenges to dosing in ECLS since patients may have multi-organ dysfunction as the half-life has been shown to be considerably prolong in excess of 3 hours for patients with hepatorenal failure (53). Beiderlinden et al, evaluated the use of argatroban in nine patients requiring ECLS. The maintenance dose in these patients was found to be 10 fold lower than the manufacturer recommended starting dose of 2 mcg/kg/min (54). However, they found no significant bleeding or thrombotic complications.
Bivalirudin is approved in the United States for use during percutaneous coronary intervention with or without HIT. Bivalirudin has some potential advantages over argatroban including a shorter half-life (~25 minutes) and predominant metabolism via a non-organ dependent pathway (proteolytic enzymes). Kiser et al, also found bivalirudin to have predictable dosing requirements in relation to renal function (55). This has led to bivalirudin being a particularly appealing option in ECLS. Pieiri et al., evaluated the use of heparin versus bivalirudin in patients requiring ECLS (49). When compared to heparin, bivalirudin was found to have a trend towards fewer bleeding and thrombosis complications but also fewer aPTT fluctuations (49). This could potentially lead to greater time within the therapeutic range for anticoagulation which is needed during ECLS especially during weaning VA ECLS. However, one must use caution with bivalirudin and ensure there is no stagnant blood in the circuit or in the heart since 80% of the metabolism is via proteolytic enzymes which could lead to clot development (56).
Direct oral anticoagulants (DOAC)
DOAC including the oral DTI and factor-Xa inhibitors (e.g., dabigatran, apixaban, rivaroxaban, etc.) have not been studied in ECLS. Given the need for oral access without the current ability to assess adequate absorption and level of anticoagulation with these drugs, this is likely to limit their use in the ECLS population (32).
Antiplatelet agents
Aspirin has been proposed as an additional agent to the anticoagulation regimen to mitigate the platelet activation that occurs during ECLS. Bein et al., evaluated the use of aspirin 1.5 mg/kg in pumpless extracorporeal lung assist and found a preservation of oxygen transfer in the membrane with no increase in bleeding. Currently there is no evidence to support or refute the addition of aspirin in ECLS (57). Despite the paucity of evidence, it is not uncommon for the adult populations requiring ECLS to have other comorbid conditions that indicate a need for antiplatelet therapy.
Conclusions
The pharmacotherapeutic considerations required in patients during ECLS presents considerable challenges well beyond the complex hemodynamic management needed. While some medication therapies can be guided through literature based evidence, clinicians are often forced to use alternative means to guide individual patient management. In the absence of greater characterization of the medications affected by ECLS, clinicians should guide speculation of drug-circuit interactions based on the physiochemical medication properties that have demonstrated reason for concern. While exceptional progress has been made to advance ECLS therapy, continued investigation into the pharmacotherapeutic management is warranted to gain a greater understanding of the implication, facilitate standardized evidence-based practices, and improve patient centered outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- ELSO Interational Summary: ECLS Registry Report. Extracorporeal Life Support Organization. Available online: . Accessed: August 1, 2016.https://www.elso.org/Registry/Statistics/InternationalSummary.aspx
- Ulldemolins M, Rello J. The relevance of drug volume of distribution in antibiotic dosing. Curr Pharm Biotechnol 2011;12:1996-2001. [Crossref] [PubMed]
- Gonzalez D, Conrado DJ, Theuretzbacher U, et al. The effect of critical illness on drug distribution. Curr Pharm Biotechnol 2011;12:2030-6. [Crossref] [PubMed]
- Green TP. The effect of extracorporeal membrane oxygenation on the biodisposition of drugs. Current Opinion in Pediatrics 1992;4:473-8.
- Dzierba AL, Abel EE. Pharmacologic challenges during mechanical circulatory support in adults. In: Erstad B, ed. Critical Care Pharmacotherapy. Lenexa, KS: American College of Clinical Pharmacy 2016:735-53.
- Wildschut ED, Ahsman MJ, Allegaert K, et al. Determinants of drug absorption in different ECMO circuits. Intensive Care Med 2010;36:2109-16. [Crossref] [PubMed]
- Mulla H, Lawson G, von Anrep C, et al. In vitro evaluation of sedative drug losses during extracorporeal membrane oxygenation. Perfusion 2000;15:21-6. [Crossref] [PubMed]
- Shekar K, Roberts JA, Mcdonald CI, et al. Protein-bound drugs are prone to sequestration in the extracorporeal membrane oxygenation circuit: results from an ex vivo study. Crit Care 2015;19:164. [Crossref] [PubMed]
- Shekar K, Roberts JA, McDonald CI, et al. Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation. Crit Care 2012;16:R194. [Crossref] [PubMed]
- Preston TJ, Ratliff TM, Gomez D, et al. Modified surface coatings and their effect on drug adsorption within the extracorporeal life support circuit. J Extra Corpor Technol 2010;42:199-202. [PubMed]
- Preston TJ, Hodge AB, Riley JB, et al. In vitro drug adsorption and plasma free hemoglobin levels associated with hollow fiber oxygenators in the extracorporeal life support (ECLS) circuit. J Extra Corpor Technol 2007;39:234-7. [PubMed]
- Rosen DA, Rosen KR, Silvasi DL. In vitro variability in fentanyl absorption by different membrane oxygenators. J Cardiothorac Anesth 1990;4:332-5. [Crossref] [PubMed]
- Unger JK, Kuehlein G, Schroers A, et al. Adsorption of xenobiotics to plastic tubing incorporated into dynamic in vitro systems used in pharmacological research--limits and progress. Biomaterials 2001;22:2031-7. [Crossref] [PubMed]
- Shekar K, Roberts JA, Smith MT, et al. The ECMO PK Project: an incremental research approach to advance understanding of the pharmacokinetic alterations and improve patient outcomes during extracorporeal membrane oxygenation. BMC Anesthesiol 2013;13:7. [Crossref] [PubMed]
- Barr J, Fraser G, Puntillo K. Clinical practice guidelines for the management of pain, agitation and delirium in adult patients in the intensive care unit. Critical Care Medicine 2013;41:263-306. [Crossref] [PubMed]
- Shapiro BA, Warren J, Egol A. Practice parameters for intravenous analgesia and sedation for adult patients in the intensive care unit: an executive summary. Society of Critical Care Medicine. Critical Care Medicine 1995;23:1596-600. [Crossref] [PubMed]
- Bhatt-Meht V, Annich G. Sedative clearance during extracorporeal membrane oxygenation. Perfusion 2005;20:309-15. [Crossref] [PubMed]
- Mehta NM, Halwick DR, Dodson BL, et al. Potential drug sequestration during extracorporeal membrane oxygenation: results from an ex vivo experiment. Intensive Care Med 2007;33:1018-24. [Crossref] [PubMed]
- Devlin JW, Roberts RJ. Pharmacology of commonly used analgesics and sedatives in the ICU: benzodiazepines, propofol, and opioids. Anesthesiol Clin 2011;29:567-85. [Crossref] [PubMed]
- Smith MT. Neuroexcitatory effect of morphine and hydromorphone: evidence implicating the 3-glucuronide metabolites. Clin Exp Pharmacol Physiol 2000;27:524-8. [Crossref] [PubMed]
- Reade MC, Finfer S. Sedation and delirium in the intensive care unit. NEJM 2014;370:444-54. [Crossref] [PubMed]
- Erstad BL, Puntillo K, Gilbert H. Pain management principles in the critically ill. Chest 2009;135:1075-86. [Crossref] [PubMed]
- Horinek EL, Kisler TH, Fish DN, et al. Propylene glycol accumulation in critically ill patients receiving continuous intravenous lorazepam infusions. Ann Pharmacother 2009;43:1964-71. [Crossref] [PubMed]
- Lemaitre F, Hasni N, Leprince P, et al. Propofol, midazolam, vancomycin and cyclosporine Therapeutic Drug Monitoring in Extracorporeal Membrane Oxygenation Circuits Primed with Whole Human Blood. Critical Care 2015;19:40. [PubMed]
- Hall RI, Sandham D, Cardinal P. Propofol vs midazolam for ICU sedation: a Canadian multicenter randomized trial. Chest 2001;119:1151-9. [Crossref] [PubMed]
- Pandharipande PP, Sanders R, Girard T. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori-designed analysis of the MENDS randomized controlled trial. Critical Care 2010;14:R38. [Crossref] [PubMed]
- Riker RR, Shehabi Y, Bokesch PM. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA 2009;301:489-99. [Crossref] [PubMed]
- Mo Y, Zimmerman AE. Role of dexmedetomidine for the prevention and treatment of delirium in intensive care unit patients. Ann Pharmacother 2013;47:869-76. [Crossref] [PubMed]
- Wagner D, Pasko D, Phillips K, et al. In vitro clearance of dexmedetomidine in extracorporeal membrane oxygenation. Perfusion 2013;28:40-6. [Crossref] [PubMed]
- Girard TD, Shintani AK, Jackson JC, et al. Risk factors for post-traumatic stress disorder symptoms following critical illness requiring mechanical ventilation: a prospective cohort study. Critical Care 2007;11:R28. [Crossref] [PubMed]
- Molina PE. Opioids and opiates: analgesia with cardiovascular, haemodynamic and immune implications in critical illness. J Intern Med 2006;259:138-54. [Crossref] [PubMed]
- ELSO Anticoagulation Guidelines 2014. Extracorporeal Life Support Organization. Available online: . Accessed: August 1, 2016.http://elsonet.org/resources/guidelines
- Esper SA, Levy JH, Waters JH, et al. Extracorporeal membrane oxygenation in the adult: a review of anticoagulation monitoring and transfusion. Anesth Analg 2014;118:731-43. [Crossref] [PubMed]
- Krueger K, Schmutz A, Zieger B, et al. Venovenous extracorporeal membrane oxygenation with prophylactic subcutaneous anticoagulation only: an observational study in more than 60 patients. Artif Organs 2017;41:186-92. [Crossref] [PubMed]
- Tomasko J, Prasad SM, Dell DO, et al. Therapeutic anticoagulation-free extracorporeal membrane oxygenation as a bridge to lung transplantation. J Heart Lung Transplant 2016;35:947-8. [Crossref] [PubMed]
- Oliver WC. Anticoagulation and coagulation management for ECMO. Semin Cardiothorac Vasc Anesth 2009;13:154-75. [Crossref] [PubMed]
- Annich GM. Extracorporeal life support: the precarious balance of hemostasis. J Thromb Haemost 2015;13 Suppl 1:S336-42. [Crossref] [PubMed]
- Aylsworth CL, Stefan F, Woitas K, et al. New technology, old standards: disparate activated clotting time measurements by the Hemochron Jr. compared with the standard Hemochron. Ann Thorac Surg 2004;77:973-6. [Crossref] [PubMed]
- Kottke-Marchant K. An algorithmic approach to hemostasis testing. Semin Thromb Hemost 2014;40:195-204. [Crossref] [PubMed]
- Adatya S, Uriel N, Yarmohammadi H, et al. Anti-factor Xa and activated partial thromboplastin time measurements for heparin monitoring in mechanical circulatory support. JACC Heart Fail 2015;3:314-22. [Crossref] [PubMed]
- Kostousov V, Nguyen K, Hundalani SG, et al. The influence of free hemoglobin and bilirubin on heparin monitoring by activated partial thromboplastin time and anti-Xa assay. Arch Pathol Lab Med 2014;138:1503-6. [Crossref] [PubMed]
- Smythe MA, Priziola J, Dobesh PP, et al. Guidance for the pratical management of heparin anticoagulants in the treatment of venous thromboembolism. J Thromb Thrombolysis 2016;41:165-86. [Crossref] [PubMed]
- Bates SM., Weitz JI. Coagulation assays. Circulation 2005;112:e53-60. [Crossref] [PubMed]
- Brill-Edwards P, Ginsberg JS, Johnston M, et al. Establishing a therapeutic range for heparin therapy. Ann Intern Med 1993;119:104-9. [Crossref] [PubMed]
- Chiu HM, Hirsh J, Yung WL, et al. Relationship between the anticoagulant and antithrombotic effects of heparin in experimental venous thrombosis. Blood 1977;49:171-84. [PubMed]
- Bembea MM, Annich G, Rycus P, et al. Variability in anticoagulation management of patients on extracorporeal membrane oxygenation: an international survey. Pediatr Crit Care Med 2013;14:e77-84. [Crossref] [PubMed]
- Whiting D, DiNardo JA. TEG and ROTEM: technology and clinical application. Am J Hematol 2014;89:228-32. [Crossref] [PubMed]
- Lee MS, Kong J. Heparin: physiology, pharmacology, and clinical application. Rev Cardiovasc Med 2015;16:189-99. [PubMed]
- Pieri M, Agracheva N, Bonaveglio E, et al. Bivalriudin versus heparin as an anticoagulant during extracorporeal member oxygenation: a case-control study. J Cardiothorac Vasc Anesth 2013;27:30-4. [Crossref] [PubMed]
- Ryerson LM, Bruce AK, Lequier L, et al. Administration of antithrombin concentrate in infants and children on extracorporeal life support improves anticoagulation efficacy. ASAIO J 2014;60:559-63. [Crossref] [PubMed]
- Byrnes JW, Sweringen CJ, Prodhan P, et al. Antithrombin III supplementation on extracorporeal membrane oxygenation: impact on heparin dose and circuit life. ASAIO J 2014;60:57-62. [Crossref] [PubMed]
- Burcham PK, Abel EE, Gerlach AT, et al. Development and implantation of a nurse-driven, sliding-scale nomogram for bivalirudin in the management of heparin-induced thrombocytopenia. Am J Health Syst Pharm 2013;70:980-7. [Crossref] [PubMed]
- Arpino PA, Hallisey RK. Effect of renal function on the pharmacodynamics of argatroban. Ann Pharmacother 2004;38:25-9. [Crossref] [PubMed]
- Beiderlinden M, Treschan T, Gorlinger K, et al. Argatroban in extracorporeal membrane oxygenation. Artif Organs 2007;31:461-5. [Crossref] [PubMed]
- Kiser TH, Burch JC, Klem PM, et al. Safety, efficacy, and dosing requirements of bivalirudin in patients with heparin induced thrombocytopenia. Pharmacotherapy 2008;28:1115-24. [Crossref] [PubMed]
- Ranucci M. Bivalirudin and post-cardiotomy ECMO: a word of caution. Crit Care 2012;16:427. [PubMed]
- Bein T, Zimmermann M, Philipp A, et al. Addition of acetylsalicylic acid to heparin for anticoagulation management during pumpless extracorporeal lung assist. ASAIO J 2011;57:164-8. [Crossref] [PubMed]