The impact of frailty on mortality after transcatheter aortic valve replacement
Introduction
Transcatheter aortic valve replacement (TAVR) is currently considered as a standard of care for patients with severe aortic stenosis (AS) who are deemed inoperable with prohibitive risk for surgical aortic valve replacement (SAVR) (1). Although TAVR is less invasive, the reported mortality in patients undergoing TAVR is considerably high (24% at 1-year and 34% at 2-year post TAVR) (1,2).
Frailty is a state of vulnerability in which patients have decreased physiologic reserve resulting in a poor outcome when a stressor is applied (3,4). This syndrome is very prevalent is reported in up to 80% of patients undergoing TAVR (5-7). Previous studies have demonstrated the adverse effects of frailty on outcomes including disability, dependency, falls, the need for long-term care facility and mortality after cardiac surgery and procedures, such as coronary artery bypass grafting, valve repair or replacement, TAVR, or combined procedures (4,8-11).
Cardiac operative risk models such as the Society of Thoracic Surgeons (STS) and the European system for cardiac operative risk evaluation (EuroSCORE) are designed to predict surgical mortality (12-14). These risk models are not specifically developed only for patients undergoing TAVR, which commonly consist of the elderly population suffering from frailty (5-7). In this review, we present the perspectives of frailty impact on mortality in patients undergoing TAVR.
Adverse effects of frailty
Frailty is defined as a state of reduced physiological reserve and impaired resistance to external stressors, resulting in compromised physical and neurocognitive function, cumulative declines across multiple organ systems and increased vulnerability to unfavorable outcomes (3,15-17). The frail phenotype includes unintentional weight loss, weakness, slow walking speeds, low albumin levels, and inability to perform the activities of daily living (ADL) (3,4). Although the frailty definition is not standardized, experts’ consensus description of this syndrome includes physical frailty comprising loss of endurance, wasting (weight loss, loss of muscle mass and strength), limited balance and mobility, diminished performance and relative inactivity and decline in cognitive function (18).
The underlying pathophysiology of frailty is thought to be aging-associated wear and tear coupled with age-related biologic changes that lead to subclinical multi-system dysfunction (16,17). Studies have demonstrated associations of frailty with dysregulation of the immune, hormonal, and endocrine systems including up-regulation of inflammatory cytokines, decreased testosterone levels, and glucose intolerance due to insulin resistance, leading to physical inactivity, malnutrition, and sarcopenia (a state of progressive decline in muscle mass and strength) (16,19). The presence of frailty has been associated with many adverse outcomes including an increased risk of falls, disability, institutionalization, health care resource use as well as mortality (8,20-23).
Frailty assessment and impact on TAVR-related outcomes
Currently, the definition of frailty is not standardized, and more than 20 instruments for measurements of frailty have been developed (24). Not surprisingly, there are a broad variety of frailty assessment tools used in published studies of patients undergoing TAVR as shown in Table 1 (1,9,25-31). Different studies used variable cutoffs and scales and some used composite scoring systems. Also, the consensus definition or gold standard instruments for physical frailty assessment have not been established. A well-standardized frailty scoring systems should be utilized in patients referred for a possible TAVR procedure (1).
Recently, the published ACC/AHA guidelines recommend the Katz ADL index, measurement of gait speed, grip strength, and muscle mass to be used for evaluation of surgical and interventional risk (32). Although the proposed assessment tools cover some aspects of frailty among surgical patients, they are not specific to patients undergoing TAVR (32).
Despite the heterogeneity of frailty assessment tools, in surgical patients, studies have shown that frailty independently prognosticates postoperative complications, hospital length of stay, and discharges to skilled nursing or assisted living facilities (33). Moreover, following cardiac surgery, a recent systematic review evaluating six studies, including 4,756 patients undergoing cardiac surgical procedures, showed that patients who were deemed frail had a higher likelihood of experiencing major adverse cardiac and cerebrovascular events, mortality, and functional decline (34). Not only in patients undergoing cardiac surgery, but studies have also shown that frail patients have worse outcomes after renal transplantation, and to be at greater risk of disability, and long hospitalization following hip fractures (33,35).
The impact of frailty on clinical outcomes following TAVR has been reported in the literature (1,9,25-31). This relationship has been described consistently despite the heterogeneity of frailty definition, use of variable cutoffs and scales, or utilization of a composite scoring system. In all of these studies, frailty has been demonstrated as an independent predictor of short and long-term mortality, procedural outcomes and longer hospital stays after TAVR as shown in Table 2.
Recently, in a multicenter study (Placement of Aortic Transcatheter Valve Trial), the role of frailty in 244 patients undergoing TAVR was evaluated. These patients deemed either high risk or inoperable for SAVR. At one year, compared with non-frail patients, mortality was significantly greater in the frail cohort (32.7% vs. 15.9%, P=0.004) (36). Additionally, Puls et al. (30) demonstrated that frailty status measured by the Katz ADL index (Table 1) in 300 patients undergoing TAVR was a robust predictor of early and late poor outcomes including mortality. Lately, Arnold et al. (37) evaluated the predictors of TAVR Poor Outcome risk models using data from the CoreValve US Pivotal Extreme and high-risk trials among 2,830 patients who underwent TAVR. Authors defined frailty as three or more deficits in the five geriatric domains (Table 1), and they found it was a significant predictor of 1-year mortality with OR of 1.42 (95% CI: 1.18–1.69) (37). These studies establish a clear relationship between adverse outcomes and frailty status before TAVR (27-30,36-38).
Frailty and prediction models of poor TAVR outcomes
Overall, high-risk or inoperable patients with severe AS who undergo TAVR, experience better outcomes including lower mortality, improved quality-of-life, and less utilization of health care resources. However, benefit from TAVR is not universal among all patients. Allocating individuals who benefit from TAVR remains to be a challenge.
Recently, a study evaluating the Medtronic CoreValve system sought to determine the patient characteristics that impact the benefits of TAVR. The authors showed an overall improvement in the quality of life and subjective symptoms after TAVR; however, a significant proportion of the enrolled cohort (39%) did not experience any benefit. Two factors were predictive of lack symptomatic improvement with TAVR including being wheelchair-bound and having low serum albumin (39). Thus, similar to the recommendation for other cardiac surgeries, frailty evaluation should be integrated into an individual risk-benefit analysis for TAVR (32,40).
Risk prediction models can help clinicians and patients understand the potential likelihood of undesirable outcomes and provide them with information that may be valuable in choosing and planning for the optimal treatment pathways. There has recently been considerable effort to improve risk assessment in TAVR (41,42). It is lately advised that the predictive performance of risk scores may be enhanced by recalibration over time and the addition of variables, intended to assess functional and cognitive capacities and frailty in the elderly (14). By combining the frailty assessment into cardiac risk evaluations, shared decision-making between physicians and patients can be improved. In the updated EuroSCORE II, poor mobility was added to the prediction model in order to provide more accuracy and reliability of high-risk classification among elderly patients (13). Besides, recent updates to the STS risk score include frailty as graded by the 5-meter walk test (43). Incorporating a frailty evaluation into an assessment of the patient risk of surgery-related morbidity or mortality promises to improve patient selection for TAVR.
A few TAVR-specific risk prediction models have recently been developed (44-49). Most studies have focused on predicting short-term and 1-year mortality. Recently, using PARTNER trial data, Arnold et al. developed the TAVR Poor Outcome risk model (combining both mortality and reduced quality of life after TAVR) (44). Subsequently, Arnold et al. externally validated their TAVR-specific risk prediction model in the CoreValve study population (37). The investigators demonstrated good performance of their 6and 12-month TAVR Poor Outcome risk models with similar moderate discrimination (c-indexes: 0.64 to 0.67) and excellent calibration (37). Additionally, in this study, the investigators only confirmed the association between frailty and poor outcomes after TAVR. Although they found adding frailty, defined as 3 or more deficits in the 5 geriatric domains, would improve the calibration and discrimination of their models, it was an only small discrimination enhancement. The TAVR Poor Outcome risk models included several factors that are correlated or classified as component or part of frailty syndrome, such as body mass index, functional status [assessed by walk test or Kansas City Cardiomyopathy Questionnaire(KCCQ)], and cognition [determined by Mini-mental state examination (MMSE)] (9,25-31). These findings suggested frailty status is important and should be considered as part of a comprehensive assessment before TAVR. Also, there is an inevitable need for a precise definition of frailty and a standardized frailty assessment tool. In addition, future studies assessing the usefulness of these models in clinical care are required.
Interventions based on frailty
We propose the incorporation of frailty into the pre, peri or postoperative period assessment in patients undergoing TAVR as shown in Figure 1. Not only screening but also preventive and therapeutic interventions are vital in frail patients. It has been noted that frail individuals benefit from early mobilization, resistance exercise, and rehabilitation (50). Frail patients may benefit from interventions in the pre, peri or postoperative period comprising intensive monitoring, early mobilization, and planned discharge to a specialized physical rehabilitation facility and exercise training (51).
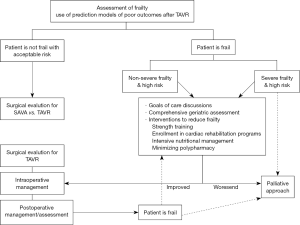
In frail patients, extended post-TAVR care such as early mobilization, intensive nutritional management and early and long-term rehabilitative programs can support a faster recovery (1). When frailty is identified in the preoperative phase, clinicians should preemptively attempt to reduce disability, weakness, and improve nutritional status before and after TAVR. Although strength training and nutritional supplementation have been the primary treatments studied to-date (19,50,52), future studies in TAVR population assessing outcomes following interventions to prevent and improve frailty are warranted. Nevertheless, in patients with severe frailty, excessive comorbidities and a reduced possibility of benefit from a TAVR might warrant a palliative approach particularly on those who are recognized by the surgical team as unsuitable candidates for an invasive treatment (53).
Conclusions
Frailty is very common in patients with severe AS undergoing TAVR and is associated with poor outcomes including mortality and reduced quality of life after TAVR. Frailty in TAVR is an active area of ongoing research. Beyond comorbidity and risk stratification, the addition of frailty as part of a comprehensive assessment before TAVR can aid appropriate patient selection for TAVR. Studies have recently added frailty scores to traditionally used risk calculators. However, there are multiple methods to measure or characterize it. The lack of standard definition of frailty makes hinders progress in understanding how frailty can be best used in risk assessment. Thus, there is a definite need for a precise definition of frailty and a gold standard assessment protocol based on well-established tests covering all aspects of frailty.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hinterbuchner L, Strohmer B, Hammerer M, et al. Frailty scoring in transcatheter aortic valve replacement patients. Eur J Cardiovasc Nurs 2016;15:384-97. [Crossref] [PubMed]
- Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012;366:1686-95. [Crossref] [PubMed]
- Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146-56. [Crossref] [PubMed]
- Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 2004;59:255-63. [Crossref] [PubMed]
- Kaul S. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014;4;371:967.
- Popma JJ, Adams DH, Reardon MJ, et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol 2014;63:1972-81. [Crossref] [PubMed]
- Finn M, Green P. Transcatheter aortic valve implantation in the elderly: who to refer? Prog Cardiovasc Dis 2014;57:215-25. [Crossref] [PubMed]
- Mathew V, Greason KL, Suri RM, et al. Assessing the risk of aortic valve replacement for severe aortic stenosis in the transcatheter valve era. Mayo Clin Proc 2014;89:1427-35. [Crossref] [PubMed]
- Afilalo J, Eisenberg MJ, Morin JF, et al. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol 2010;56:1668-76. [Crossref] [PubMed]
- Green P, Woglom AE, Genereux P, et al. Gait speed and dependence in activities of daily living in older adults with severe aortic stenosis. Clin Cardiol 2012;35:307-14. [Crossref] [PubMed]
- Sündermann S, Dademasch A, Praetorius J, et al. Comprehensive assessment of frailty for elderly high-risk patients undergoing cardiac surgery. Eur J Cardiothorac Surg 2011;39:33-7. [Crossref] [PubMed]
- Shroyer AL, Coombs LP, Peterson ED, et al. The Society of Thoracic Surgeons: 30-day operative mortality and morbidity risk models. Ann Thorac Surg 2003;75:1856-64; discussion 1864-5.
- Nashef SA, Sharples LD, Roques F, et al. EuroSCORE II and the art and science of risk modelling. Eur J Cardiothorac Surg 2013;43:695-6. [Crossref] [PubMed]
- Webb J, Rodés-Cabau J, Fremes S, et al. Transcatheter aortic valve implantation: a Canadian Cardiovascular Society position statement. Can J Cardiol 2012;28:520-8. [Crossref] [PubMed]
- Sintek M, Zajarias A. Patient evaluation and selection for transcatheter aortic valve replacement: the heart team approach. Prog Cardiovasc Dis 2014;56:572-82. [Crossref] [PubMed]
- Finn M, Green P. The Application of Frailty to the Modern Cardiac Risk Assessment: a Case-Based Review. Curr Cardiovasc Risk Rep 2015.9. [PubMed]
- Chen MA. Frailty and cardiovascular disease: potential role of gait speed in surgical risk stratification in older adults. J Geriatr Cardiol 2015;12:44-56. [PubMed]
- Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013;14:392-7. [Crossref] [PubMed]
- Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol 2014;63:747-62. [Crossref] [PubMed]
- Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet 2013;381:752-62. [Crossref] [PubMed]
- Rochat S, Cumming RG, Blyth F, et al. Frailty and use of health and community services by community-dwelling older men: the Concord Health and Ageing in Men Project. Age Ageing 2010;39:228-33. [Crossref] [PubMed]
- Ensrud KE, Ewing SK, Taylor BC, et al. Frailty and risk of falls, fracture, and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci 2007;62:744-51. [Crossref] [PubMed]
- Shamliyan T, Talley KM, Ramakrishnan R, et al. Association of frailty with survival: a systematic literature review. Ageing Res Rev 2013;12:719-36. [Crossref] [PubMed]
- de Vries NM, Staal JB, van Ravensberg CD, et al. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev 2011;10:104-14. [Crossref] [PubMed]
- Seiffert M, Sinning JM, Meyer A, et al. Development of a risk score for outcome after transcatheter aortic valve implantation. Clin Res Cardiol 2014;103:631-40. [Crossref] [PubMed]
- Sündermann SH, Dademasch A, Seifert B, et al. Frailty is a predictor of shortand mid-term mortality after elective cardiac surgery independently of age. Interact Cardiovasc Thorac Surg 2014;18:580-5. [Crossref] [PubMed]
- Green P, Woglom AE, Genereux P, et al. The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: a single-center experience. JACC Cardiovasc Interv 2012;5:974-81. [Crossref] [PubMed]
- Stortecky S, Schoenenberger AW, Moser A, et al. Evaluation of multidimensional geriatric assessment as a predictor of mortality and cardiovascular events after transcatheter aortic valve implantation. JACC Cardiovasc Interv 2012;5:489-96. [Crossref] [PubMed]
- Schoenenberger AW, Stortecky S, Neumann S, et al. Predictors of functional decline in elderly patients undergoing transcatheter aortic valve implantation (TAVI). Eur Heart J 2013;34:684-92. [Crossref] [PubMed]
- Puls M, Sobisiak B, Bleckmann A, et al. Impact of frailty on shortand long-term morbidity and mortality after transcatheter aortic valve implantation: risk assessment by Katz Index of activities of daily living. EuroIntervention 2014;10:609-19. [Crossref] [PubMed]
- Kamga M, Boland B, Cornette P, et al. Impact of frailty scores on outcome of octogenarian patients undergoing transcatheter aortic valve implantation. Acta Cardiol 2013;68:599-606. [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg 2014;148:e1-e132. [Crossref] [PubMed]
- Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 2010;210:901-8. [Crossref] [PubMed]
- Sepehri A, Beggs T, Hassan A, et al. The impact of frailty on outcomes after cardiac surgery: a systematic review. J Thorac Cardiovasc Surg 2014;148:3110-7. [Crossref] [PubMed]
- Woods NF, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc 2005;53:1321-30. [Crossref] [PubMed]
- Green P, Arnold SV, Cohen DJ, et al. Relation of frailty to outcomes after transcatheter aortic valve replacement (from the PARTNER trial). Am J Cardiol 2015;116:264-9. [Crossref] [PubMed]
- Arnold SV, Afilalo J, Spertus JA, et al. Prediction of Poor Outcome After Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 2016;68:1868-77. [Crossref] [PubMed]
- Alfredsson J, Stebbins A, Brennan JM, et al. Gait Speed Predicts 30-Day Mortality After Transcatheter Aortic Valve Replacement: Results From the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Circulation 2016;133:1351-9. [Crossref] [PubMed]
- Osnabrugge RL, Arnold SV, Reynolds MR, et al. Health status after transcatheter aortic valve replacement in patients at extreme surgical risk: results from the CoreValve U.S. trial. JACC Cardiovasc Interv 2015;8:315-23. [Crossref] [PubMed]
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS), Vahanian A, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451-96. [Crossref] [PubMed]
- Wong CY, Green P, Williams M. Decision-making in transcatheter aortic valve replacement: the impact of frailty in older adults with aortic stenosis. Expert Rev Cardiovasc Ther 2013;11:761-72. [Crossref] [PubMed]
- Reynolds MR, Hong JC. What We Are Learning From Transcatheter Aortic Valve Replacement Risk Prediction Models. J Am Coll Cardiol 2016;68:1878-80. [Crossref] [PubMed]
- Tang GH, Lansman SL, Cohen M, et al. Transcatheter aortic valve replacement: current developments, ongoing issues, future outlook. Cardiol Rev 2013;21:55-76. [Crossref] [PubMed]
- Arnold SV, Reynolds MR, Lei Y, et al. Predictors of poor outcomes after transcatheter aortic valve replacement: results from the PARTNER (Placement of Aortic Transcatheter Valve) trial. Circulation 2014;129:2682-90. [Crossref] [PubMed]
- Capodanno D, Barbanti M, Tamburino C, et al. A simple risk tool (the OBSERVANT score) for prediction of 30-day mortality after transcatheter aortic valve replacement. Am J Cardiol 2014;113:1851-8. [Crossref] [PubMed]
- Iung B, Laouénan C, Himbert D, et al. Predictive factors of early mortality after transcatheter aortic valve implantation: individual risk assessment using a simple score. Heart 2014;100:1016-23. [Crossref] [PubMed]
- Debonnaire P, Fusini L, Wolterbeek R, et al. Value of the "TAVI2-SCORe" versus surgical risk scores for prediction of one year mortality in 511 patients who underwent transcatheter aortic valve implantation. Am J Cardiol 2015;115:234-42. [Crossref] [PubMed]
- Edwards FH, Cohen DJ, O'Brien SM, et al. Development and Validation of a Risk Prediction Model for In-Hospital Mortality After Transcatheter Aortic Valve Replacement. JAMA Cardiol 2016;1:46-52. [Crossref] [PubMed]
- Hermiller JB Jr, Yakubov SJ, Reardon MJ, et al. Predicting Early and Late Mortality After Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 2016;68:343-52. [Crossref] [PubMed]
- Fiatarone MA, O'Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med 1994;330:1769-75. [Crossref] [PubMed]
- Binder EF, Yarasheski KE, Steger-May K, et al. Effects of progressive resistance training on body composition in frail older adults: results of a randomized, controlled trial. J Gerontol A Biol Sci Med Sci 2005;60:1425-31. [Crossref] [PubMed]
- Ottenbacher KJ, Ottenbacher ME, Ottenbacher AJ, et al. Androgen treatment and muscle strength in elderly men: A meta-analysis. J Am Geriatr Soc 2006;54:1666-73. [Crossref] [PubMed]
- Lauck S, Garland E, Achtem L, et al. Integrating a palliative approach in a transcatheter heart valve program: bridging innovations in the management of severe aortic stenosis and best end-of-life practice. Eur J Cardiovasc Nurs 2014;13:177-84. [Crossref] [PubMed]




