Three-dimensional (3D) printed endovascular simulation models: a feasibility study
Introduction
Three-dimensional (3D) printing is a manufacturing process more accurately referred to as additive manufacturing. An object is created by sequentially printing layer upon layer guided by a computer file. There are several key additive manufacturing technologies currently available, including stereolithography, fused-deposition modeling and selective laser sintering. Stereolithography makes use of the property of a group of liquid compounds called photopolymers which become solidified when exposed to a light source such as a laser.
Modern imaging modalities such as computed tomography (CT) allow patient-specific anatomy to be processed and manipulated in order to generate a file that can be read by a 3D printer, commonly referred to as a surface tessellation language (STL) file. Once sent to a 3D printer, an object can be printed in a variety of materials including plastics, metals, and ceramics.
The scientific literature regarding 3D printing of models or devices for medical purposes is currently sparse. So far, applications of 3D printing in medicine have predominantly focused on anatomic modeling and surgical planning in prosthetic reconstructive surgery (1-3). Despite media reports highlighting unique cases of 3D printed skull and mandibular reconstructions, this is not yet common practice (4,5). Paediatric cardiac surgery is another area where 3D printing of heart models has been used to enhance and facilitate communication with parents of children with congenital heart defects (6). In an era where medicine needs to be increasingly cost effective, 3D printing remains a promising technology. At present, however, there are limited quality quantitative data to support its use.
Vascular applications of 3D printing lag behind its orthopedic and cardiac surgical counterparts from both an anatomic modeling and implant perspective. Theoretically, vascular devices such as stents might be able to be 3D printed but the implant of a fully 3D printed vascular device has yet to be realised. We sought to determine the feasibility of 3D printing to produce a model of the human aorta along with its major branch vessels for the purpose of bench-top simulation of endovascular procedures.
Endovascular procedures require a high degree of precision and skill that has traditionally been taught exclusively through an apprenticeship model based entirely in patients. More recently endovascular simulation with high fidelity virtual simulation (VR) has gained prominence and been educationally validated (7). Another option for endovascular training is cadaveric simulation, though this approach is expensive and often impractical (8). The traditional mantra of ‘see one, do one, teach one’ is now being replaced by ‘see one, sim many, do one’ (9). Since both VR and cadaveric endovascular simulation are associated with high financial cost and need for ongoing technical support, we sought to develop a simple, low cost, maintenance free, endovascular simulator to improve training and address many of the barriers to more wide-spread adoption of simulation training (10).
Methods
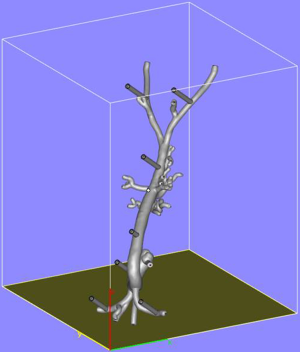
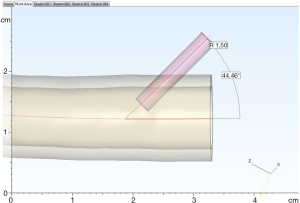
A CT angiogram of the aorta from aortic arch to common femoral arteries was imported in digital imaging and communications in medicine (DICOM) format into dedicated post-processing software (Mimics® Innovation Suite, Materialise NV, Leuven, Belgium). The data were processed to reduce imaging noise and segment the anatomy of interest. The aorta and its major branches were isolated from the images via thresholding and interactive editing operations. Subsequently, a 3D surface model (STL file) was created. The 3-matic® software was then used to smooth the surface of the blood vessels, select wall thickness and add stabilization poles to the model so that it could be attached securely to a Plexiglas baseplate. Finally a hole was incorporated into each common femoral artery anteriorly in a 45-degree angle to accommodate the external diameter of a 6-Fr endovascular sheath (Figure 1), mimicking real-life arterial access. Before producing the physical model, the contours of the final model were overlaid on the medical images to ensure the model remained anatomically correct.
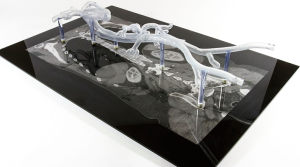
The aortic model was printed using stereolithography whereby the model was built up by curing a resin with ultraviolet (UV) light. In preparation for printing, the model was oriented to minimize printing time and dedicated software (e-stage, Materialise NV, Leuven, Belgium) used to automatically calculate the required support material needed to stabilize the model during the printing process (Figure 2). This file was uploaded to the 3D printer and the model printed layer-by-layer. The final model consisted of 5,240 layers of 0.1 mm with a build time of 96 hours and 38 minutes. Once printing had been completed (Figure 3), the supporting structures were removed and an UV light resistant polish/varnish applied (Figure 4) to produce a transparent finish (Figure 5). Finally the model was screwed to the Plexiglas baseplate (Figure 6). The resultant model was lightweight (2.7 kg) and portable.


To further replicate real-life feel and enable the use of hydrophilic wires, the model was then filled with standard tap-water and sealed with two screw caps. A 6-Fr sheath was inserted into each common femoral artery (Figure 7) and catheters and guidewires inserted through the sheath in the standard fashion.
To assess the utility of the model as an endovascular simulator, opinions were sought from 96 physicians (1–7 years post-medical qualification). The physicians were given 15 minutes to use the model with the help of an experienced endovascular practitioner (either an interventional radiologist or vascular surgeon). The tasks performed are outlined in Table 1. Candidates were also given the opportunity to compare the 3D printed model with an endovascular virtual reality (VR) simulator, so that comparison could be made to an educationally validated training tool. Data were collected using a 5-point Likert scale.

Full table
Results
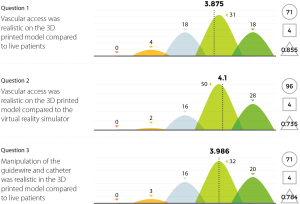
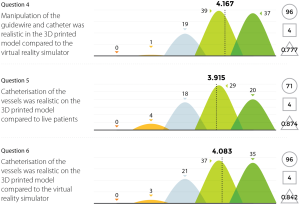
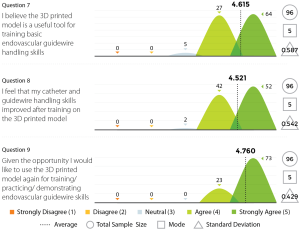
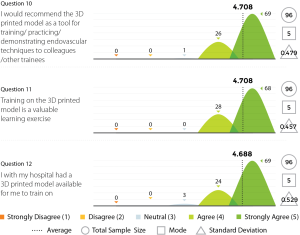
Three experienced endovascular practitioners initially undertook each of the tasks outlined in Table 1 on the 3D printed model confirming their feasibility. All procedures were performed through the standard 6 Fr sheath. A total of 96 candidates undertook procedures on VR simulators and the 3D printed model. Following this training they completed a post-trial questionnaire rating their agreement with 12 statements regarding their training experience on a standard 5-point Likert scale. Not all questions were relevant to all candidates, for example, if the candidate had no experience in performing endovascular procedures in live patients then the questions regarding comparison between live patients and the 3D model would not have been answered. Results are summarized in Figures 8-11.
Discussion
3D printing is a technology that has been available since the 1980s, but only recently has it seen larger scale adoption in the manufacturing community. This is mainly due to improvements in software and an expanding array of available 3D printers. The parallel growth of medical imaging, in particular CT has resulted in a symbiotic potential as CT data (DICOM files) can be imported into 3D printing software.
So far the largest medical application of 3D printing has been in procedural planning. Several case reports suggest a 3D printed model might help a surgeon better understand the anatomy before a procedure and consequently result in an improved surgical outcome (11-13). Large scale data is currently lacking to support this claim and new applications for 3D printing in medicine are rapidly evolving. Medical education could stand to benefit greatly as 3D printing could allow the rapid creation of anatomically accurate prototypes for a range of educational purposes. One such example is a 3D printed temporal bone model that was created for surgical training (14).
Current approaches to endovascular simulation include VR simulation and cadaveric training, whilst there are data to educationally validate these technologies, the barriers of high financial investment and ongoing technical support continue to limit their use. This means endovascular training continues to take place on patients, placing them at unnecessary risk when existing technologies exist to train early stage endovascular skills. 3D printing can solve this problem as it can produce a comparatively low cost simulator requiring minimal maintenance whereby key catheter and guidewire skills can be learned before attempting a procedure on a patient. By performing the skills through a sheath in an anatomically accurate model in addition to the skills learned in Table 1, the concepts of pushability, torquability and trackability are experienced first-hand. Additional skills not included in Table 1 which could be performed with the model are iliac angioplasty and the use of an endovascular snare.
The Likert data presented in this feasibility study supports the educational value of the 3D printed endovascular simulator. While individual trainee perceptions are a subjective measure, they represent the first evidence of its kind for a 3D printed simulation model. Future studies could seek to educationally validate the model through studies of direct comparison with alternative simulation methods or real procedures.
This model has several limitations which will hopefully be addressed with future versions. Firstly, how can the anatomic accuracy of the model be verified? The software used to create the initial STL file was the medical version of Mimics Innovation Suite which has been Federal Drug Administration (FDA, USA) approved and Conformité Européenne (CE) marked. The initial isolation of the vasculature for the CT was performed by an experienced Mimics Innovation Suite user with input from a radiologist. Before printing the model, the proposed 3D data was overlaid on the original CT to confirm anatomic accuracy. Secondly, the model is created in a resin material called Tusk XC2700T which when exposed to UV light becomes solidified. The model is therefore rigid and this might negatively influence the feel of the catheter and guidewire as elasticity of a vessel wall is not replicated. The option of 3D printing in silicone was explored to enhance flexibility, however, current 3D printed silicone is not transparent and it is tearable which would limit its durability. Finally, in order to remain water tight to allow the use of hydrophilic guidewires, the model cannot be opened fully therefore a stent cannot be deployed and retrieved inside the model. Future model designs may be able to address this issue.
Conclusions
This study has demonstrated the feasibility of a 3D printed vascular model for simulation and training purposes. Initial feedback using Likert scales provides the first evidence of its kind for a 3D printed medical model, although greater educational validation is needed. The presented model is lower cost than existing VR endovascular simulators, requires less technical maintenance, is fully portable and provides a realistic training experience that compares favourably to VR and real patients affording an opportunity to learn key endovascular skills while experiencing enhanced haptics including pushability, torquability and trackability. By addressing the barriers of existing endovascular simulation options, a 3D printed model might be able to achieve larger scale usage. The limitations in flexibility and closed design of the model may be addressed in future versions. Overall, 3D printing of endovascular simulation models has the opportunity to improve endovascular simulation and consequently may benefit patient safety.
Acknowledgements
We would like to thank Materialise (Materialise NV, Leuven, Belgium) and in particular Tine Putseys for the technical support in creating this 3D printed model. The model described in this manuscript was funded by VIRThinkTank Ltd.
Footnote
Conflicts of Interest: S Mafeld, C Nesbitt, J McCaslin, A Bagnall, P Davey and R Williams are part of VIRThinkTank Ltd. including being shareholders. The aforementioned authors are currently considering marketing the model described in the manuscript.
References
- Cohen J, Reyes SA. Creation of a 3D printed temporal bone model from clinical CT data. Am J Otolaryngol 2015;36:619-24. [Crossref] [PubMed]
- Eltorai AE, Nguyen E, Daniels AH. Three-Dimensional Printing in Orthopedic Surgery. Orthopedics 2015;38:684-7. [Crossref] [PubMed]
- Schwam ZG, Chang MT, Barnes MA, et al. Applications of Three-dimensional Printing in Facial Plastic Surgery. J Oral Maxillofac Surg 2016;74:427-8. [Crossref] [PubMed]
- Borreli L. Chinese Girl Becomes World’s First To Receive Full Skull Reconstruction Via 3D Printing [Internet]. 2015. Cited 2015 Dec 6. Available online: http://www.medicaldaily.com/chinese-girl-becomes-worlds-first-receive-full-skull-reconstruction-3d-printing-343390
- Transplant jaw made by 3D printer claimed as first - BBC News [Internet]. 2012. Cited 2015 Dec 6. Available online: http://www.bbc.co.uk/news/technology-16907104
- Biglino G, Capelli C, Wray J, et al. 3D-manufactured patient-specific models of congenital heart defects for communication in clinical practice: feasibility and acceptability. BMJ Open 2015;5:e007165. [Crossref] [PubMed]
- Rudarakanchana N, Van Herzeele I, Desender L, et al. Virtual reality simulation for the optimization of endovascular procedures: current perspectives. Vasc Health Risk Manag 2015;11:195-202. [Crossref] [PubMed]
- Chevallier C, Willaert W, Kawa E, et al. Postmortem circulation: a new model for testing endovascular devices and training clinicians in their use. Clin Anat 2014;27:556-62. [Crossref] [PubMed]
- Lee JT, Peruzzaro A, Krummel T, et al. SS22. See One, Sim One, Do One, Teach One: Results of a Prospective Randomized Trial of Endovascular Skills Training for Surgical Residents. J Vasc Surg 2012;55:27S. [Crossref]
- Nelson K, Bagnall A, Nesbitt C, et al. Developing cross-specialty endovascular simulation training. Clin Teach 2014;11:411-5. [Crossref] [PubMed]
- Wu XB, Wang JQ, Zhao CP, et al. Printed three-dimensional anatomic templates for virtual preoperative planning before reconstruction of old pelvic injuries: initial results. Chin Med J (Engl) 2015;128:477-82. [Crossref] [PubMed]
- Kiraly L, Tofeig M, Jha NK, et al. Three-dimensional printed prototypes refine the anatomy of post-modified Norwood-1 complex aortic arch obstruction and allow presurgical simulation of the repair. Interact Cardiovasc Thorac Surg 2016;22:238-40. [Crossref] [PubMed]
- Krauel L, Fenollosa F, Riaza L, et al. Use of 3D Prototypes for Complex Surgical Oncologic Cases. World J Surg 2016;40:889-94. [Crossref] [PubMed]
- Hochman JB, Kraut J, Kazmerik K, et al. Generation of a 3D printed temporal bone model with internal fidelity and validation of the mechanical construct. Otolaryngol--Head Neck Surg Off J Am Acad Otolaryngol- Head Neck Surg 2014;150:448-54. [Crossref]