Extracorporeal life support during cardiac arrest and cardiogenic shock—how good is the evidence really?
For emergency physicians and cardiologists, patients with refractory cardiac arrest or cardiogenic shock complicating myocardial infarction are children of sorrow: mortality is extremely high, and not much improvement can be reported for the last decades. At least the beneficial effect of early revascularization was clarified by the SHOCK-Trial (1), with an estimated risk reduction (30-days/1-year-mortality) amounting to about 18% [relative risk (RR) 0.82 (95% CI 0.69–0.97)] by primary percutaneous intervention/primary coronary artery bypass grafting (2). Nevertheless mortality still remains high. No wonder that not only drugs but also mechanical support devices like the intraaortic balloon pump (IABP) or pumps like the Impella or the TandemHeart are in the therapeutic scope, to increase cardiac output and thereby transiently stabilize haemodynamics (2,3). With the use of extracorporeal life support (ECLS)/veno-arterial extracorporeal membrane oxygenation (VA-ECMO), not only cardiac function can be supported, but also pulmonary function. All these devices have been used for a long time despite a lack of evidence from randomized controlled trials (RCTs), only based on recommendations by experts and our confidence in pathophysiology, reckoning that an increase in cardiac output should improve survival.
However, after presentation of the IABP-SHOCK II trial in 2012/2013 (4,5) with neutral results of IABP in patients with myocardial infarction complicated by cardiogenic shock, IABP use in Germany dropped nearly by half, from about 8,500 in 2012 to about 5,000 in 2014 (6). Vice versa, the application numbers for other percutaneous mechanical support devices increased, for instance, for VA-ECMO in Germany from about 500 in 2012 to about 3,000 in 2014 (6).
This dramatic increase, however, is alarming, because all these instrumentations are not based on firm evidence, but rather on “pathophysiology-driven common sense”. An increase in cardiac output and oxygenation by ECLS in refractory cardiac arrest and cardiogenic shock is mandatory, but does not guarantee an improved survival of these patients.
In view of this uncertainness due to the lack of RCTs, we really welcome the systematic review and meta-analysis of extracardiac life support during cardiac arrest and cardiogenic shock complicating myocardial infarction by Ouweneel et al. (7). This publication comes from the group of Professsor Josef P.S. Henriques from Amsterdam, highly renowned in this field. The authors systematically searched MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials and the published subset of PubMed updated to December 2015. Nine of the 13 studies—all cohort studies and no RCTs at all—reported about cardiac arrest patients (n=3,098) and four included patients with cardiogenic shock after acute myocardial infarction (n=235). Analysis included data pooling by a Mantel-Haenzel random effects model, and heterogeneity was examined by the I2 statistic. In refractory cardiac arrest (Table 1), the use of ECLS was associated with an absolute increase of 30-day survival of 13% compared with patients in whom ECLS was not used [95% CI: 6–20%; P<0.001; numbers needed to treat (NNT): 7.7], and it was also associated with a higher rate of favourable neurological outcome at 30 days (absolute risk difference 14%; 95% CI: 7–20%; P<0.0001; NNT: 7.1). Similar results showed propensity matched analysis, including five studies and 438 patients (219 in both groups). In cardiogenic shock (Table 1), ECLS showed a 33% higher 30-day survival compared with IABP (absolute risk difference 0.33; 95% CI: 0.14–0.52; P<0.001; NNT: 3), but no difference when compared with Impella/TandemHeart (−0.03; 95% CI: −0.21 to 0.14; P=0.70; NNH: 33) and also no significant difference to the pooled data (0.14; 95% CI: −0.08 to 0.35; P=0.20; NNT: 7.1). Only two studies reported complications, amounting to about 15% with regards to peripheral vessels complications/leg ischaemia, including 3% of compartment syndromes. From their findings the authors concluded that (I) in cardiac arrest, the use of ECLS was associated with an increased survival rate as well as an increase in favourable neurological outcome and (II) in the setting of cardiogenic shock there was an increased survival with ECLS compared with IABP.

Full table
We can accept these conclusions in case of cardiac arrest patients, but in case of patients with cardiogenic shock complicating myocardial infarction, we have to solve a problem: we can agree that ECLS is better than IABP, as we know that IABP does not lower 30-day mortality in these patients (4). On the other hand, the authors also showed that ECLS is not better than device treatment with Impella/TandemHeart (Table 1). However, nobody yet has shown that treatment with Impella/TandemHeart improves prognosis in patients with cardiogenic shock complicating myocardial infarction. So, when treatment with Impella/TandemHeart is not proven and treatment with ECLS is not better than Impella/TandemHeart use, we still do not know—as the authors state—whether ECLS treatment indeed improves survival in those patients.
Can we “trust” the positive results of the presented meta-analysis? For us, the results of this meta-analysis with respect to refractory cardiac arrest are more convincing than those for cardiogenic shock. They further support the resuscitation guidelines (8,9), which give a “weak recommendation with very-low-quality evidence” and suggest that “ECPR” (extracorporeal cardiopulmonary resuscitation) “is a reasonable rescue therapy for selected patients with cardiac arrest when initial conventional CPR is failing in settings where this can be implemented” (8).
In principle, a word of scepticism is always indicated whenever results of meta-analyses are solely based on non-randomized trials. And this, indeed, is especially the case concerning the results propagated for patients with cardiogenic shock complicating myocardial infarction, as all these studies are cohort studies moreover with only small numbers of patients (Tables 1,2).
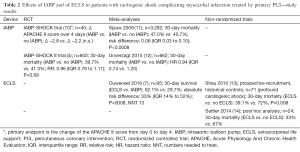
Full table
ECLS may be beneficial for patients with CS, but for which patient at which point of time? To answer these questions not only hemodynamic instability has to be taken into account, but also the degree of systemic inflammation (SIRS) and consecutive established microcirculatory disturbances. A clinical approximation to this question might be the early calculation of appropriate scores to estimate the severity of multi-organ dysfunction or even failure. We could demonstrate the prognostic value of the APACHE II score in patients with infarct related cardiogenic shock (10). And we are convinced that there will be patients who will benefit from the means of ECLS, but we are in need for valid instruments to identify these patients. The answers to the questions given above could also help to choose the best of the available devices for the individual patient.
So, can we really “trust” the work presented by Henriques-team? We think, we can! In 2009, the Henriques team published a systematic review and meta-analysis of the role of IABP in STEMI patients (11) (Table 2). And based on their neutral findings, the authors provocatively asked in their title: “Should we change the guidelines?” At that time, the use of IABP in STEMI patients with cardiogenic shock was a class I recommendation in the guidelines. We, at that time, did the IABP SHOCK trial (10), a small randomized trial with 40 STEMI patients with cardiogenic shock complicating myocardial infarction. We could not see any improvement in the severity of disease—as measured by the APACHE II score—in these patients by the IABP. And then, in the follow-up trial—the IABP SHOCK II trial (4,5) with 600 patients with cardiogenic shock complicating myocardial infarction, no reduction in mortality could be observed by the use of IABP (Table 2). So, the Henriques-team had been right with their meta-analysis finding 3 years before. The guidelines nowadays state that IABP is not routinely recommended in cardiogenic shock (III/B) (15).
The recent meta-analysis of the Henriques-team (7) about ECLS during cardiac arrest and cardiogenic shock has not a neutral result as in case of the IABP-meta-analysis (11), but a positive one. But does this mean that we now should routinely apply ECSL in refractory cardiac arrest and in cardiogenic shock? The answer is clearly “no”, as even a good meta-analysis as those reported by Ouweneel et al. (7) is not enough for recommendation requiring a RCT with an endpoint “survival”. And a prospective, randomized, controlled ECLS trial in patients with refractory cardiac arrest as well as in patients with cardiogenic shock complicating myocardial infarction could indeed be done: the IABP-SHOCK II trial with 600 patients—exclusively included in a single country (Germany) —was the proof of principle!
Following the line of the IABP-SHOCK trial and the IABP-SHOCK II trial: we eagerly await an “ECLS-ARREST” trial and an “ECLS-SHOCK” trial!
Acknowledgements
The authors gratefully acknowledge stimulating discussions with members (S Nuding, J Schröder, T Otto and L Thieme) of the TEMPHUS project team FKZ: I3GW0034B. The TEMPHUS project (FKZ: I3GW0034B) about temporary mechanical support is sponsored by the German Ministry of Education and Research (BMBF).
Footnote
Conflicts of Interest: K Werdan, formerly Director of the Department of Medicine III of the University Hospital Halle (Saale) Germany, is now working in the Department of Medicine III as scientist of the TEMPHUS project team (FKZ: I3GW0034B) about temporary mechanical support, sponsored by the German Ministry of Education and Research (BMBF). R Prondzinsky has no conflicts of interest to declare.
References
- Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med 1999;341:625-34. [Crossref] [PubMed]
- Thiele H, Ohman EM, Desch S, et al. Management of cardiogenic shock. Eur Heart J 2015;36:1223-30. [Crossref] [PubMed]
- Werdan K, Gielen S, Ebelt H, et al. Mechanical circulatory support in cardiogenic shock. Eur Heart J 2014;35:156-67. [Crossref] [PubMed]
- Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287-96. [Crossref] [PubMed]
- Thiele H, Zeymer U, Neumann FJ, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomized, open-label trial. Lancet 2013;382:1638-45. [Crossref] [PubMed]
- Karagiannidis C, Brodie D, Strassman S, et al. Extracorporeal membrane oxygenation: evolving epidemiology and mortality. Intensive Care Med 2016;42:889-96. [Crossref] [PubMed]
- Ouweneel DM, Schotborgh JV, Limpens J, et al. Extracorporeal life support during cardiac arrest and cardiogenic shock: a systematic review and meta-analysis. Intensive Care Med 2016;42:1922-34. [Crossref] [PubMed]
- Soar J, Callaway CW, Aibiki M, et al. Part 4: Advanced life support. 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation 2015;95:e71-e120. [Crossref] [PubMed]
- Soar J, Nolan JP, Böttiger BW, et al. European Resuscitation Council Guidelines for Resuscitation 2015 Section 3. Adult advanced life support. Resuscitation 2015;95:100-47. [Crossref] [PubMed]
- Prondzinsky R, Lemm H, Swyter M, et al. Intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: the prospective, randomized IABP SHOCK Trial for attenuation of multiorgan dysfunction syndrome. Crit Care Med 2010;38:152-60. [Crossref] [PubMed]
- Sjauw KD, Engström AE, Vis MM, et al. A systematic review and meta-analysis of intra-aortic balloon pump therapy in ST-elevation myocardial infarction: should we change the guidelines? Eur Heart J 2009;30:459-68. [Crossref] [PubMed]
- Unverzagt S, Buerke M, de Waha A, et al. Intra-aortic balloon pump counterpulsation (IABP) for myocardial infarction complicated by cardiogenic shock. Cochrane Database Syst Rev 2015.CD007398. [PubMed]
- Sheu JJ, Tsai TH, Lee FY, et al. Early extracorporeal membrane oxygenator-assisted primary percutaneous coronary intervention improved 30-day clinical outcomes in patients with ST-sement elevation myocardial infarction complicated with profound cardiogenic shock. Crit Care Med 2010;38:1810-7. [Crossref] [PubMed]
- Sattler S, Khaladi N, Zaruba MM, et al. Extracorporal life support (ECLS) in acute ischaemic cardiogenic shock. Int J Clin Pract 2014;68:529-31. [Crossref] [PubMed]
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129-200. [Crossref] [PubMed]


