Cannulation techniques for extracorporeal life support
Extracorporeal life support (ECLS) is an umbrella term including various modalities of temporary mechanical cardiopulmonary assistance of the failing heart and/or lungs. The main objective of ECLS is to provide systemic perfusion and gas exchange allowing the heart and lungs to rest and recover or bridge the patient to another modality of mechanical support or transplantation (1-6). The technology involves redirecting the blood from the body through cannulas and connecting tubing to gas-exchange membrane and then returning it by means of a pump back to the patient’s circulation. Several modes of ECLS are available for advanced heart/lung failure. The exact configuration of the circuit and cannulation strategy depends on the organ which needs to be supported: cardiovascular (VA-ECMO, bridge to short term BiVAD or long term LVAD support), respiratory (VV-ECMO, extracorporeal CO2 removal—ECCO2R) or both (VA-ECMO), as well as clinical context: emergent extracorporeal cardiopulmonary resuscitation (ECPR) involving percutaneous cannulation at the bedside or elective surgical procedure via sternotomy (central ECMO). In this article we will give an overview of cannulation techniques for different types of extracorporeal support, technical considerations, pitfalls and potential complications.
ECMO
Extracorporeal Membrane Oxygenation involves centrifugal pump, heat exchanger and oxygenator. It can provide both circulatory and respiratory support and is therefore used in severe heart, lung or combined heart-lung failure. The most commonly sites for percutaneous cannulation for establishing peripheral ECMO are femoral artery, femoral vein or internal jugular vein. In central ECMO right atrium and aorta are the preferred vessels.
There are two separate configurations of ECMO circuit: veno-venous (VV) and veno-arterial (VA). Both types of ECMO involve inserting two cannulas—one for draining the blood from venous system (SVC/IVC) to ECMO circuit, the other one—for returning the oxygenated blood either to RA (VV) or to arterial system (VA). Therefore the main objective of VV-ECMO is to maintain gas exchange (oxygenation and removal of CO2) in isolated lung failure and preserved cardiac output. During VA-ECMO oxygenated blood is returned into systemic circulation thus fulfilling two functions—gas exchange and circulatory support. The distinct feature of peripheral VA ECMO is that the oxygenated blood is delivered to aorta via femoral artery in retrograde fashion and competes with native antegrade circulation generated by the heart. Consequently, it bears inherent potential problems: separate perfusion of the lower and upper part of the body (watershed phenomenon), distention of the LV, and resulting pulmonary oedema due to increased afterload produced by ECMO. The latter requires close monitoring and fine adjustment of the flows, peripheral vascular resistance, vasopressor support and oxygenation. More advanced configuration of peripheral VA-ECMO employing three cannulas can be used to optimize cardiorespiratory support and deal with aforementioned problems: VVA and VAV ECMO. Extracorporeal life support organization (ELSO) guidelines contain recommendation on establishing, maintaining and weaning ECMO (1).
Central VA ECMO is a separate entity. The technique is very invasive as it requires chest opening, and sometimes the chest may remain open for a few days. Therefore the risks of bleeding, infection, and injury to vessels are greater. It is only used for patients who will imminently die. The advantage of this technique is that it can provide the best perfusion flow and offload the left ventricle, but a major disadvantage is that it bypasses the lungs and the heart. If the blood flow from the pulmonary circulation is completely diverted there is a significant risk of thrombosis (2).
The different modes of ECMO support require different types of cannulae. These also require different connectors and securing techniques. The main goal of ECMO cannulation is to provide the least traumatic and most durable and simplified method of delivering the blood to and from the circuit. Detailed knowledge of the blood flow and possible pitfalls of each technique is essential when choosing cannulation site. Experience in using the variety of extracorporeal cannulation cannot be overrated. Training is essential before embarking on such highly traumatic support. In the rest of the article we will discuss the types of cannulation and available cannulae for the different modes of extracorporeal support.
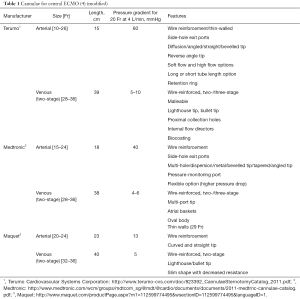
Central ECMO cannulation
Central ECMO (Figure 1) involves sternotomy and direct surgical cannulation of the right atrium and aorta. Institution of central ECMO occurs in theatre and requires a full surgical team (surgeon, anaesthetist, scrub nurse). The main advantages of this type of ECLS are good venous drainage and reliable arterial return to the proximal aorta in antegrade fashion. It allows full control over left ventricular decompression by placing a vent, usually via right superior pulmonary vein (LV apex being another, albeit less frequently used, option). The size of the cannulae is defined by body surface area the calculated ECMO flow necessary to achieve metabolic requirements of the patient. Usually, for aortic cannulation we use 22–24 Fr, and venous cannulation in the form of two stage cannula, ranging from 32–34 to 40–46 Fr. It is of utmost importance to secure the cannulae in their position to prevent any bleeding from cannulation site or their displacement during the following care on ICU. We normally use multilevel measures (purse string, snuggers, spigots, fixing the cannulae to the chest wall from within the chest cavity, Hollister anchors to fix the cannulae to the skin) to make sure that cannulae are held in place since the consequence of displacement can be devastating for the patient. At the end of the procedure the chest can either be left open with occlusive dressing or closed. The latter requires tunnelling the cannulae through soft tissues via separate incisions. We are aiming to avoid skin sutures as it causes significant discomfort to awake patients and source of infection and bacterial colonisation.
Central ECMO cannulation can be used as a first-choice modality for cardiorespiratory support (postcardiotomy shock being the most typical indication) or as an upgrade from peripheral configuration when it does not allow to achieve adequate flows to provide good end-organ function or overcome problems inherent to peripheral ECMO: LV distention and differential hypoxia of the heart or the brain.
Invasive nature of the central cannulation for ECMO support is its main disadvantage. Sternotomy related issues (bleeding, infection, resternotomy), aortic dissection, inability to mobilize the patient if the chest was left open, ischaemic events of embolic origin are potential complications of this cannulation technique (3). The blood flow largely bypasses the pulmonary circulation and there is a risk of thrombosis; if aortic valve is not opening there is a risk of thrombus formation on the top of the aortic valve. In case of expected prolonged central VA-ECMO support, the cannulas can be tunnelled sub-xyphoid, allowing formal approximation of the sternotomy edges. Some units might favour waking up and mobilising the patient. Major advantages are: highest flow rates suitable even for patient needing supraphysiological flows; supports both oxygenation and circulation; can be done as an emergency; suitable for post-cardiotomy cardiorespiratory failure; perfuses all parts of the body. It can be used for interhospital transfer in isolated circumstances, however, the risk of cannulae dislodgement and bleeding is high.
Many companies manufacture cannulae for cardiopulmonary bypass, and these can also be used for central ECMO. Wire re-inforced cannulae are advantageous as they avoid kinking. Longer cannulae allow easier skin tunnelling. When placing arterial cannulae they need to be secured in position, which offers minimal endothelial vessel damage. Biocompatible coating and side holes are helpful in avoiding such damage.
Commercially available cannulae for central VA ECMO are summarized in Table 1.
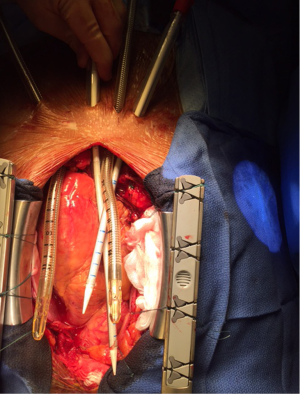
BiVAD cannulation
Sometimes central ECMO is used for initial stabilisation of a patient who would not survive post cardiotomy separation from CPB or after cardiac arrest resuscitation. Occasionally, the chest remains opened and/or packed with swabs. When the patient is stabilised the chest can be closed and central ECMO can be changed to BiVADS. This circuit is devoid of oxygenator and hence provides only circulatory support. The cannulation for BiVADS requires drainage of blood from the right atrium or ventricle and return to the pulmonary artery and in parallel drainage of blood from the left ventricle and return to the aorta. Cannulae used for this are the same as for central ECMO. In case when there is recovery of the right ventricle, the patient is bridged to a long term LVAD. When the patient cannot be weaned from BiVADS, they can be bridged towards heart transplantation.
In contrast to ECMO, BiVAD configuration involves cannulating the chambers of the heart (usually apex of RV and LV, but RA or LA also possible) (5). Although it makes the procedure technically more challenging, it provides an excellent means of unloading the heart and maintaining the blood flow via pulmonary vasculature. All four cannulae are tunnelled through soft tissues (Figure 2) and chest is closed allowing reasonable degree of mobilisation on ICU. Moreover, should the lung function deteriorate, an oxygenator can be incorporated into RVAD circuit providing both circulatory and respiratory assistance. Therefore BiVAD is the modality of choice for medium term (weeks/months) mechanical circulatory support.
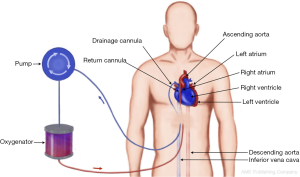
Peripheral VA-ECMO
During VA-ACMO oxygenated blood is returned to the arterial part of systemic circulation using peripheral cannulation via femoral, axillary or carotid (infants) artery. This configuration of the circuit bypasses the lungs and heart and therefore provides both respiratory and circulatory support. From functional point of view it acts as a heart-lung machine allowing support of the functions of these organs completely by providing calculated full flow up to 2.5 L/m2/min (Figure 3).
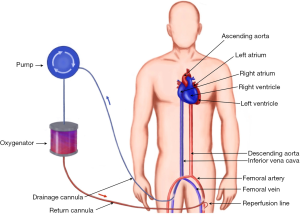
Indications for VA-ECMO include: refractory cardiogenic shock (myocardial infarction, failure to wean from cardiopulmonary bypass after cardiac surgery, fulminant myocarditis, decompensated chronic heart failure, peripartum cardiomyopathy), as a bridge to durable LVAD or transplantation, as a part of resuscitation in certain circumstances, as a bridge to recovery following lung transplantation for pulmonary hypertension (6).
Cannulation
The cannulation can be performed either using percutaneous Seldinger technique, open cutdown, open cutdown Seldinger (“semi Seldinger”) or open cutdown with end-to-side graft (Dacron) to the artery. The venous drainage is provided by the cannula (19–25 Fr) in the femoral or internal jugular vein the tip of which is positioned at the level of the right atrium. Flow rates are more often limited by the size of the venous (inflow) drainage cannula than the arterial (outflow) cannula (7).
The most commonly arteries used for arterial return are femoral, axillary (adults) or common carotid artery (neonates and children weighing less than 15 kg) (8). The most common artery used for peripheral VA-ECMO is the femoral artery. Axillary cannulation provides easier ambulation, lower risk of limb ischaemia, antegrade flow, potentially higher cerebral oxygen saturation, decreased risk of atheroembolisation, but has higher rate of bleeding and requires surgical approach to establish vascular access (9).
The arterial return is directed into descending aorta via arterial cannula (15–23 Fr). The size of the cannula depends on calibre of the vessels and flows required to achieve adequate circulatory support. The following formula could be used to choose the diameter of the cannula (D) in French gauge system (10):
Commercially available cannulae for peripheral VA ECMO are summarized in Table 2.
One of the complications of retrograde flow return into femoral artery is lower limb ischaemia. The reasons for this are 2 fold: the femoral arterial lumen is partially occluded by the cannula, and the retrograde flow redirects the little remaining native femoral blood flow in a retrograde direction. The consequence is that the distal femoral arterial tree has minimal or no blood flow. One method of overcoming this is using a reperfusion circuit: (first described in 1995 (11) inserted into femoral artery distal to main arterial cannula which allows selective perfusion of the lower limb and prevents its ischaemia. Another method is perfusion of the lower limb by cannulating tibial artery (12). The limb perfusion should be monitored very carefully: clinically and using Doppler ultrasound.
Since oxygenated and decarboxylated blood is returned into the arterial system in retrograde fashion it creates unique haemodynamic phenomena of special clinical significance: LV distension and interaction of blood flows from the heart and ECMO circuit.
Despite the fact that venous blood is drained to ECMO circuit, LV still receives some blood from pulmonary and bronchial circulation, i.e., not fully unloaded. Retrograde flow creates additional afterload to LV, which can lead to LV distention, increased wall stress as a consequence of elevated end-diastolic pressures, reduced coronary flow, pulmonary oedema and subsequently hypoxaemia. The extent of this potential problem varies and depends on degree of impairment of LV, extreme manifestation being absence of aortic valve opening, stasis of blood and potentially thrombus formation in LV. That is why it is crucial to monitor the presence of pulsatility of the arterial trace confirming ejection of blood by LV in patients on peripheral ECMO.
There are several options available to deal with this pitfall: reducing afterload and improving myocardial contractility by optimizing vasopressor and inotropic support; ensuring optimal position of the venous cannula for best possible drainage; fluid offloading by diuretics or CVVH; the use of IABP to reduce afterload (although there is a concern that it may interfere with arterial return from ECMO circuit) (13); if AR present reducing ECMO flows rather than increasing may be beneficial as it creates better conditions for LV ejection; atrial septostomy; direct mechanical venting of the LV using either trans-aortic or transapical routes; use of percutaneous LVADs like Impella or TandemHeart to drain LV or LA respectively.
A more advanced option is to upgrade veno-arterial to veno-venous-arterial configuration of ECMO circuit (VVA) inserting a second cannula for venous blood drainage (usually via internal jugular vein). The enhanced drainage reduces preload for LV, thus minimizing the risk of its distension (14).
The second pathophysiological feature of peripheral ECMO is related to its retrograde flow competing with native circulation generated by the heart. This can lead to situation when upper and lower parts of the body receive blood supply from different sources. It becomes clinically relevant when lungs are severely impaired and LV receives unoxygenated blood. Resultant arterial hypoxemia in the upper part of the body (and cyanotic appearance of it) has been known as Harlequin or North-South syndrome (15). For that reason, sampling from the right radial artery is warranted to monitor level of oxygenation of blood perfusing the heart and brain. However this approach is not reliable when watershed point is located very proximal in the aorta (Figure 4) (14).
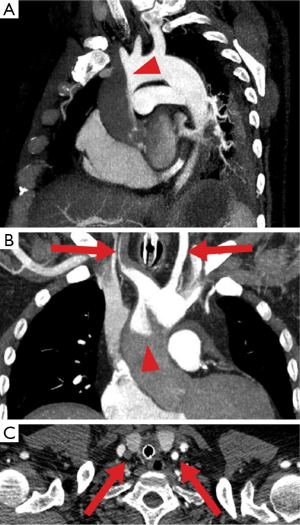
The options to overcome this problem are: adjusting ventilatory settings (FiO2, PEEP, recruitment manoeuvres); optimizing lung function (diuretics, draining effusion and so on); upgrading the circuit to either VVA ECMO as described above (reduces the return of unoxygenated blood to the left heart) or veno-arterial-venous ECMO (VAV) during which the return of oxygenated blood to the body is split into two: (I) to the aorta and (II) to the RA. This configuration functions as combination of VV (pushes oxygenated blood through the lungs leading to better oxygen delivery to the heart and brain) and VA ECMO (delivers oxygenated blood to the systemic circulation). Since pulmonary circulation is lower resistance part of the circulation there is a risk of preferential diversion of split arterial return toward the lungs rather than to systemic circulation. It requires close registering differential flows and partial occlusion of the inflow tubing to pulmonary circulation may be needed to achieve an optimal distribution of flows between two inflow limbs (16).
The concept of flow competition or differential hypoxia is only relevant when LV is ejecting poorly oxygenated blood. If either LV does not eject or lung function is preserved, the phenomenon of dual circulation becomes less clinically important.
Circulatory support
Both blood pressure and total flow (cardiac output + ECMO flow) are important for adequate organ perfusion. Centrifugal pumps (most frequently used in peripheral ECMO circuits) are preload and afterload dependant. The flow may drop either due to decreased inflow (hypovolaemia, thrombosis, tension pneumothorax, pericardial clots) or increased afterload (mechanical obstruction of the cannula, increased SVR, high arterial pressure). Optimization of the flow is achieved by fine tuning of these three variables: preload, afterload and pump speed (RPM). Careful adjustment of vasopressor or vasodilatory support allows maintaining mean arterial blood pressure in the optimal range of 65–90 mm Hg. The flow rate should be set at the level sufficient for oxygen delivery and organ perfusion but not too high to prevent LV from ejecting by excessive afterload (can lead to distension and thrombosis of LV and aortic root). The presence of pulsatility of the arterial waveform is the easiest way of confirming that LV output is preserved. Regular ECHO studies can serve the same purpose and confirm aortic valve opening as well as to monitor the process of the recovery of cardiac function and guide through the process of weaning from ECMO.
Duration and weaning
There is little evidence behind the optimal duration of ECMO support and best time for weaning. Departmental protocols and individual clinical needs dictate the timeframe and withdrawal technique. Potential benefit of continuing ECMO in hope of end organ function improvement and cardiac recovery should be weighed against the risk of complications inherent to this method of mechanical circulatory support (bleeding, thrombosis, vascular dissection and rupture, limb ischaemia, cardiac thrombosis, coronary hypoxia). It is generally accepted that one week is usually enough to optimize organ function and proceed to either to decannulation (cardiac recovery) or bridging to more durable support (VAD or heart transplantation) (1,17).
VV-ECMO cannulation
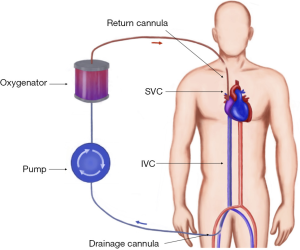
During VV-ECMO (Figure 5) the blood is drained into the circuit, where it is oxygenated and carbon dioxide is removed, following which it is returned to the patient’s venous system. This configuration is therefore relies on the patient’s own circulation and requires adequate cardiac function. It is used in isolated failure of the lungs unresponsive to optimal ventilatory support and medical treatment. According to ELSO guidelines the indications for VV-ECMO include (18): hypoxic respiratory failure with PaO2/FiO2 <100 mmHg and/or Murray score 3–4; CO2 retention despite full mechanical ventilatory support; severe air leak syndromes; deteriorating patient on a lung transplantation list; immediate respiratory collapse (asphyxia, pulmonary embolism).
Majority of the patients supported by VV-ECMO recover respiratory function, but this technique can also be used to bridge patients to lung transplantation. It should not be used in multi-organ failure, terminal illness irrecoverably neurological insult.
Evidence
The ongoing international multicenter randomized Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome (EOLIA) trial (ClinicalTrials.gov Identifier: NCT01470703) will test the efficacy of early VV-ECMO in patients with severe ARDS. It is expected to overcome weaknesses of previous trials [CESAR trial (19)] with better control of mechanical ventilation in the control group, timing of ECMO onset, and strict adherence to randomization procedures (20).
Cannulation
The traditional cannulation technique involves cannulating femoral vein for the drainage of the blood and internal jugular vein for venous return. Large bore cannulas (23–31 Fr) are used for venous drainage whereas smaller (15–19 Fr) are sufficient for venous return. The tips of the cannulas should be at the level of atrial-caval junctions (14). The correct position of the cannulas is very important to prevent recirculation of the oxygenated blood between two cannulas. Therefore the placement is usually facilitated by echocardiography and fluoroscopy. Another combination utilises femoral approach by draining the blood with a shorter cannula from the IVC, and returning it directly to the right atrium. Recirculation can be more problematic with this technique, but it avoids neck vessels cannulation and injury.
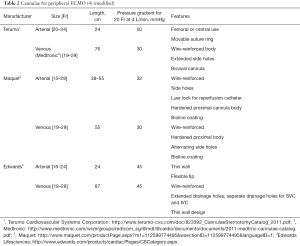
The alternative way of establishing vascular access for VV-ECMO is the use of a double lumen bicaval cannula (AVALON ELITE® Bi-Caval Dual Lumen Catheter, Maquet). It has three ports: proximal, middle and distal. The distal port located at the tip of the cannula and should be positioned just below atrial-IVC junction—it drains the blood from IVC. The proximal port drains the blood from SVC. The middle port rests in the RA and faces tricuspid valve. This port is designed to return oxygenated blood from ECMO circuit (21). The benefits of the Avalon cannula include reduction of bleeding risks since only one vessel is punctured, lower rate of recirculation, and ease of mobilisation. The similar principle of using a single cannula for both drainage and return is implemented in Paraglide Dual Lumen Cannula (Chalice, Nottinghamshire, UK) with drainage lumen significantly larger than the one for reinfusion. Commercially available dual lumen VV ECMO cannulae are summarized in Table 3.
Complications
The biggest problem with VV ECMO support is inadequate flow provision. Not infrequently patients in extreme respiratory failure are septic and have supraphysiological cardiac output. VV ECMO flows are limited to 5–6 L/min and may not oxygenate sufficient proportion of the circulating volume. Hence the patient may still suffer from hypoxaemia despite VV ECMO support. Adding a second drainage cannula may ameliorate flow but carries additional risk of vessel damage and clotting. Other significant risks during VV ECMO support are: oxygenated blood recirculation (avoided by using dual lumen cannula and meticulous single cannula positioning), vessel injury, thrombosis, and infection.
Extracorporeal CO2 removal (ECCO2R)
Indications for ECCO2R are hypercapnic respiratory failure states. As CO2 is readily removed from the blood the flows required are lower than flows for providing oxygenation. Therefore, small cannulae (14–24 Fr), low flows (<1 L/min) are sufficient. Cannulating any central vein is sufficient and the most commonly utilized one is the internal jugular vein. Integrated systems offering this kind of support are: Hemolung Respiratory Assist System (ALung Technologies, Pittsburgh, PA, USA), ilA active (Novalung, Hechingen, Germany), and Hemodec DECAPsmart (Hemodec, Salerno, Italy) and a pumples configuration (AV-ECMO) Novalung (Novalung GmbH, Hechingen, Germany). Flow is driven by patient’s cardiac output. It can reach 2.5 L/min—decarboxylation is the main goal but partial oxygenation also possible (22).
Evidence
The large randomized Strategy of Ultra Protective lung ventilation with Extracorporeal CO2 Removal for New-Onset moderate to severe ARDS (SUPERNOVA) trial is currently underway, which will test the benefits of early tidal volume and plateau pressure reduction allowed by the latest generation ECCO2R device (PALP, MAQUET; ILA-active, NOVALUNG; Hemolung, ALung) in patients with moderate forms of ARDS (20).
PA-LA cannulation
The pumpless configuration can also be established using central cannulation via sternotomy. It involves diversion the blood from pulmonary artery to gas exchange mebrane with low resistance (Novalung, Maquet Quadrox-iD) and returning the blood to the left atrium (23). The most common indication for this type of respiratory support is bridge to lung transplantation in patients with pulmonary hypertension. Among the advantages of this paracorporeal support is ability to provide decarboxylation, oxygenation, RV remodelling and greater scope for ambulation (22,24,25).
Conclusions
Extracorporeal support for critically ill patients is a growing area. Although its safety is constantly improving with technology and experience it is selected only for the extremely ill patients who are unlikely to survive without this. Cannulation for extracorporeal support is one of the major sources of morbidity. Detailed knowledge of the patient circulation and the extracorporeal support needed, as well as experience are important to offer the best support option and to avoid complications.
Acknowledgements
We would like to thank Miss Anna Valchanova for creating the ECMO diagrams. Anna Valchanova: diagram design; Barbora Parizkova: intraoperative photo.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Extracorporeal life support organization (ELSO guidelines). 2013. Available online: http://www.elso.org/resources/Guidelines.aspx
- Murphy DA, Hockings LE, Andrews RK, et al. Extracorporeal membrane oxygenation-hemostatic complications. Transfus Med Rev 2015;29:90-101. [Crossref] [PubMed]
- Saeed D, Stosik H, Islamovic M, et al. Femoro-femoral versus atrio-aortic extracorporeal membrane oxygenation: selecting the ideal cannulation technique. Artif Organs 2014;38:549-55. [Crossref] [PubMed]
- Kohler K, Valchanov K, Nias G, et al. ECMO cannula review. Perfusion 2013;28:114-24. [Crossref] [PubMed]
- Schlensak C, Schibilsky D, Siepe M, et al. Biventricular cannulation is superior regarding hemodynamics and organ recovery in patients on biventricular assist device support. J Heart Lung Transplant 2011;30:1011-7. [Crossref] [PubMed]
- Tudorache I, Sommer W, Kühn C, et al. Lung transplantation for severe pulmonary hypertension--awake extracorporeal membrane oxygenation for postoperative left ventricular remodelling. Transplantation 2015;99:451-8. [Crossref] [PubMed]
- Schaheen BW, Thiele RH, Isbell JM. Extracorporeal life support for adult cardiopulmonary failure. Best Pract Res Clin Anaesthesiol 2015;29:229-39. [Crossref] [PubMed]
- Stulak JM, Dearani JA, Burkhart HM, et al. ECMO cannulation controversies and complications. Semin Cardiothorac Vasc Anesth 2009;13:176-82. [Crossref] [PubMed]
- Biscotti M, Bacchetta M. The "sport model": extracorporeal membrane oxygenation using the subclavian artery. Ann Thorac Surg 2014;98:1487-9. [Crossref] [PubMed]
- Conrad S. Vascular access for ECLS. In: Extracorporeal Life Support for Adults. Humana Press 2016:268. Available online: http://www.springer.com/gp/book/9781493930043
- Greason KL, Hemp JR, Maxwell JM, et al. Prevention of distal limb ischemia during cardiopulmonary support via femoral cannulation. Ann Thorac Surg 1995;60:209-10. [Crossref] [PubMed]
- Spurlock DJ, Toomasian JM, Romano MA, et al. A simple technique to prevent limb ischemia during veno-arterial ECMO using the femoral artery: the posterior tibial approach. Perfusion 2012;27:141-5. [Crossref] [PubMed]
- Schroeter T, Vollroth M, Höbartner M, Dhein S, Sahlisch S, Borger MA, et al. Extracorporeal Membrane Oxygenation and Intra-Aortic Ballon Pump – an appropriate combination or useless battle of materials? Thorac Cardiovasc Surg 2013;61:OP19. [Crossref]
- Napp LC, Kühn C, Hoeper MM, et al. Cannulation strategies for percutaneous extracorporeal membrane oxygenation in adults. Clin Res Cardiol 2016;105:283-96. [Crossref] [PubMed]
- Chung M, Shiloh AL, Carlese A. Monitoring of the adult patient on venoarterial extracorporeal membrane oxygenation. ScientificWorldJournal 2014;2014:393258.
- Scott K, Schmidt B. Modes of ECLS. Extracorporeal Life Support for Adults. Humana Press, 2016:269.
- Rousse N, Juthier F, Pinçon C, et al. ECMO as a bridge to decision: Recovery, VAD, or heart transplantation? Int J Cardiol 2015;187:620-7. [Crossref] [PubMed]
- ELSO Adult Respiratory Failure Supplement to the ELSO General Guidelines [Internet]. 2013. Available online: http://www.elso.org/resources/Guidelines.aspx
- Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63. [Crossref] [PubMed]
- Schmidt M, Hodgson C, Combes A. Extracorporeal gas exchange for acute respiratory failure in adult patients: a systematic review. Crit Care 2015;19:99. [Crossref] [PubMed]
- Shaheen A, Tanaka D, Cavarocchi NC, et al. Veno-Venous Extracorporeal Membrane Oxygenation (V V ECMO): Indications, Preprocedural Considerations, and Technique. J Card Surg 2016;31:248-52. [Crossref] [PubMed]
- Reeb J, Olland A, Renaud S, et al. Vascular access for extracorporeal life support: tips and tricks. J Thorac Dis 2016;8:S353-63. [Crossref] [PubMed]
- Hoganson DM, Gazit AZ, Boston US, et al. Paracorporeal lung assist devices as a bridge to recovery or lung transplantation in neonates and young children. J Thorac Cardiovasc Surg 2014;147:420-6. [Crossref] [PubMed]
- Mayes J, Niranjan G, Dark J, et al. Bridging to lung transplantation for severe pulmonary hypertension using dual central Novalung lung assist devices. Interact Cardiovasc Thorac Surg 2016;22:677-8. [Crossref] [PubMed]
- Patil NP, Mohite PN, Reed A, et al. Modified technique using Novalung as bridge to transplant in pulmonary hypertension. Ann Thorac Surg 2015;99:719-21. [Crossref] [PubMed]