Antibody darts on target for acute myelogenous leukemia
In the last few years immunotherapy for malignant disease has moved into the mainstream of therapeutic development. In the field of hematologic malignancies powerful antibody therapies have successfully been used to target B-lymphocyte malignancies. Antibodies have been used alone, but can be engineered linking them to toxins or to T lymphocytes, either joined to the zeta chain of the T cell receptor (chimeric antigen receptors), or through bi-specific antibodies binding T cells by anti CD3 to the lymphoid target through, for example the anti CD19 variable portion [reviewed in (1)]. Clinical trials clearly confirm the power of strategies which bring T cells with their cytotoxic potency into close proximity with surface antigen targets on the malignant cell (2-4).
In contrast to these exciting advances in the immunotherapy of lymphoid malignancies and acute lymphoblastic leukemia, immunotherapy for acute myelogenous leukemia (AML) has yet to be applied as successfully. AML has always been a daunting disease to treat. Apart from the identification of a small percentage of individuals with good risk features who respond to specific therapeutic approaches and specific treatment strategies, no substantial improvements in therapy have been achieved for decades for the majority of individuals developing AML (5,6). While chemotherapy can achieve durable remissions in younger patients with favorable risk leukemia characteristics, individuals over 60 years, who represent the majority of patients with AML and the closely associated disease myelodysplastic syndrome (MDS), have poorer outcomes with leukemia-free survivals measured more frequently in months not years. Stem cell transplantation (SCT) in remission is the treatment of choice for standard and high risk AML and MDS but reduced intensity regimens, applicable to older patients, confer a higher risk of disease relapse (7). It is generally agreed that improvements in AML outcome cannot be achieved by further modifications to chemotherapy and that new approaches are needed, tolerable in older patients, and specifically targeting the leukemia. This need drives research into targeted therapies and to immunotherapy in particular.
That AML is susceptible to immune control and eradication by immune cells is substantiated by the curative power of allogeneic SCT delivering a graft-versus-leukemia effect through the donor’s T cells and NK cells (8). A current question is whether the patient’s own immune response can be similarly directed to eliminate leukemia, thus avoiding the need for a transplant. It is clear that AML interacts with the immune system, with the result that an otherwise leukemia-directed T cell attack is suppressed by AML—a process referred to as immune editing (9,10). AML suppresses T cell proliferation through a number of mechanisms: T cells in AML express the checkpoint markers PD1 and Tim3 rendering them susceptible to apoptosis by PD1-L on the leukemia. Several tumor-associated antigens (TAA) are expressed by AML and T cells recognising PRAME and WT1 can be found in the blood of patients in remission (11). However whether these TAA are central to a functional immune response to the leukemia is not known.
A therapeutic strategy which bypasses the need to know the precise TAA targets is to follow the approach successfully applied to lymphoid malignancies and redirect T cells to AML by targeting a surface antigen with an antibody based molecule. Figure 1 illustrates the various approaches under development. Anti CD33 coupled to an immunotoxin has efficacy in AML, but toxicity curtailed commercial development (12). Bi-specific antibodies to CD33 and CD123 have been also engineered but not tested clinically (15). CD33 is also expressed by normal myeloid progenitors and treatment with anti CD33 is limited by prolonged cytopenia caused by attrition to the common myeloid progenitors (12). In a recent publication Chichili and colleagues (17) describe the preclinical development and validation of a bispecific antibody [Dual-Affinity Re-Targeting (DART)] which binds to the interleukin-3 receptor CD 123 on the surface of AML blasts and uses anti CD3 to capture cytotoxic T cells and bring them into contact with leukemia (19). Several criteria will determine the clinical success of this approach: (I) the design of the bispecific antibody which affects binding to the target and the effector cell, its in vivo distribution and fate; (II) the quality of the antigen target; (III) adverse side effects from off-target effects—in particular damage to other myeloid tissues and cytokine release syndromes (CRSs) from activated T cells. Here we evaluate how the CD123/DART measures up to these desiderata.
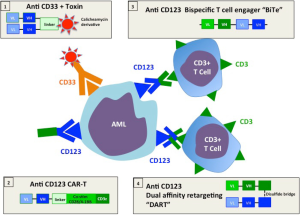
Antibody design
A critical feature of artificial constructs linking T cells to the target is the construction of the antibody and its linkage to the T cell. Figure 1 compares the constructs currently in the process of clinical translation and early clinical trials that link T cells to their targets either through bispecific antibodies or through chimeric constructs involving the T cell receptor. The first bispecific antibody to be developed commercially and used in clinical trials was the bi-specific T-cell engager (BiTE) (20). Recombinant technology is used to link the variable heavy and light domains of two antibodies as a single 55 kDa polypeptide chain. In clinical trials the CD19xCD3 BiTE blinatumomab has shown efficacy in early trials of relapsed lymphomas (1,2). In a separate development the MacroGenics company developed a DART bispecific antibody (20). This format consists of two polypeptide chains each bearing the separated variable domains of heavy and light chains of the two antigen binding specificities (see Figure 1). The construct is not readily denatured because polypeptides are stabilized through a C-terminal disulfide bridge. In a murine study CD19 (B cell) specific DARTs outperformed BiTEs with superior B-cell lysis and specific T cell activation (21). Thus among the strategies under development, DARTs may have superior qualities over BiTEs. When compared with chimeric T cell constructs (CARs) DARTs have the advantage that the requirement for in vitro T cell gene transduction is avoided (13,14). To optimize the physical properties of the chimeric antibody for AML treatment the antibody design ensures that the CD123/DART is more avid for the leukemic cell than the T cell, ensuring that T cells are preferentially bound by antibodies that have reached their target. This may reduce the unwanted activation of unbound T cells which would both reduce the therapeutic potential of the DART and risk unwanted side effects.
The quality of CD123 as an antibody target (Figure 2)
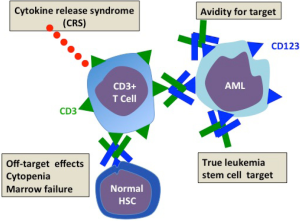
The ideal AML surface antigen should be well expressed on leukemic cells but not expressed on normal myeloid cells and particularly their progenitors. Conversely on the leukemic cell, the antigen should be present on the leukemic progenitors responsible for disease relapse after chemotherapy-induced remission. The antigen should not be downregulated by the malignancy as a means of immune escape from antibody targeting. Among a very limited field of myeloid surface antigens, CD123 the interleukin 3 receptor (IL3R) emerges as the best antibody target. As reviewed in (16) the expression of CD123 in normal and leukemic hematopoietic lineages has been well investigated in normal adult marrow, blood, cord blood and fetal liver and in leukemia. CD123 is strongly expressed on myeloid and B lymphoid precursors but absent or low on erythrocyte and megakaryocyte precursors. The earliest hematopoietic progenitors reside within the CD34+CD38 negative hematopoietic stem cell (HSC) compartment. Such cells are capable of long-term hematopoietic repopulation in irradiated immune deficient mice. Some but not all of CD34 cells express CD123 suggesting the possibility that antibodies to CD123 might spare at least some of the earliest normal progenitors. Consistent with this are studies which showed that IL3R antibodies reduced but did not prevent repopulation of human hematopoiesis in immune deficient mice (22). CD123 has been extensively studied in AML. CD123 is found in all AML subtypes (and interestingly on B lymphoblastic leukemias). In fact IL-3Rα overexpression on AML blasts was associated with poor prognosis features such as Flt3 ligand mutations, unfavorable karyotype, and failure to achieve remission (23). Fortuitously there is often aberrant overexpression of CD123 on CD34+CD38– AML cells. Such cells are true leukemic stem cells (LSC) initiating and maintaining leukemia in immunodeficient mice. In their paper Chichili et al. (17) performed preclinical testing of the CD123/DART (currently named MGD006) in the macaque. They showed that the in the monkey humanized antibodies to CD123 and CD3 recognized respectively hematopoietic cells and T cells in a similar tissue distribution to man. CD123/DART is a small molecule which is rapidly removed from the circulation with a half-life of about 30 minutes. They therefore gave MGD006 by continuous infusion to the macaques in increasing doses up to 100 ng/kg every 4 days with 3-day rest. Lastly the highest treatment group received a continuous infusion for 7 days. Although, especially after the first dose, there was a temporary rise of IL6 (the cytokine that best characterizes CRS with T cell based therapy), the infusions appear to have been tolerated. Circulating CD3 cells and CD3 cells fell rapidly. The fall in CD123 was sustained, only recovering slowly after the 4 weeks of therapy finished. CD3 cells, however, rebounded after each infusion cycle. Although neutrophils, platelets and hematocrit fell during the infusion period only the hematocrit fell below the normal range and all values recovered within weeks of stopping infusion. No monkey developed bone marrow failure. To test the in vivo therapeutic potential of the MGD006 to co-opt human T cells to AML lines they first showed that T cells with CD123/DART induced a dose-dependent killing of AML cell lines and primary AML blasts. To explore in vivo antileukemia effects they established subcutaneous tumors of the myeloid line KG-1a and another AML line, administered human peripheral blood mononuclear cells (PBMC) and gave a continuous intraperitoneal infusion of MGD006. The group receiving PBMC and CD123/DART showed significant tumor regression while the control groups receiving CD123/DART alone or PBMC alone showed tumor progression. Subsequently this group further explored the therapeutic potential of the CD123/DART. They studied the interaction between T cells, antibody and primary human AML targets and cell lines in vitro and in vivo in non-obese diabetic immune deficient (NSG) mice (18). They confirmed that the CD123 DART binds to both human CD3 and CD123 to mediate target-effector cell association, T-cell activation, proliferation, and receptor diversification. The CD123/DART also induces a dose-dependent killing of AML cell lines and primary AML blasts in vitro. Using stromal cells, which maintain survival of primary AML blasts, they were still able to achieve significant cytotoxicity with the T cell DART combination. They then established leukemia lines of K562 cells transduced with CD123 and a photofluorescent dye in NSG mice and tested the ability of the DART with T cells to control the leukemia. They showed by bioluminescent imaging that the leukemia signal was reduced to that of non-leukemia controls in mice that had received DART and T cells. In the same model they were also able to achieve “near-complete” elimination of leukemia derived from primary AML samples, with very low T cell doses (effector target ratios of 1:100). Together the studies of this group confirm both the safety and efficacy of the DART approach and established the basis for dosing and schedule in a human trial. In the leukemia models the in vivo results demonstrating powerful T cell cytotoxicity induced by the bispecific antibody are particularly promising for their clinical potential.
The safety of CD123/DART (Figure 2)
Immediate adverse reactions to antibody infusion depend on the degree to which the immune response is contained in proximity to the leukemia. Adverse effects can come from cytokine release most likely to occur in bulky disease causing a life-threatening CRS (24). In preclinical safety trials the macaque non-human primate is one of the most frequently used. For assessing CD123/DART antibody kinetics and safety the macaque is an appropriate model: compared with humans the hematopoietic system has a similar distribution of CD123 on myeloid and B cell lineages. Encouragingly the researchers showed that CD123/DART was safe in primates with no prolonged cytopenia or bone marrow failure. Of particular importance is the ability of the non-human primate to predict CRSs induced by T cell activation on site with CD123 bearing targets. Data from the macaque trials indicated that CD123 DART was well tolerated. Based on these findings approval has been given for human studies. While preclinical safety has been shown, it is no substitute for a cautious dose finding/dose escalation study in man. A phase I trial in patients with AML assessing safety pharmacokinetics and activity in the CD123/DART has been initiated and is currently underway.
What is the future of antibody therapy for AML? A minimum would be the demonstration of control of otherwise resistant disease. While dramatic remissions might be induced by CD123/DART, the leukemia is notorious for its ability to become resistant to therapy by clonal selection from the highly diverse leukemic population. Resistance could take the form of evolution of clones weakly expressing CD123, or those that successfully edit the immune response (9,10). Whether antibody-directed CD3 cells can avoid leukemic suppression through myeloid-derived suppressor cell-like function, recruitment of regulatory T cells, production of tumor necrosis factor-α or STAT3 based inhibition remains to be seen (25). Such constraints will decide how DARTs can be used. As remission therapy by further reducing residual disease they could pave the way for relapse free survival after allogeneic SCT. Alternatively, it may be possible to potentiate their antileukemic effect by checkpoint inhibitors which reduce the induction of T cell apoptosis by AML expressing PDL1/2 (9). Lastly they may inform future more efficient yet safe design of next generation bispecific antibodies which could achieve the so far unattained goal of safe but potent antileukemic therapy to replace toxic chemotherapy, much needed for the older AML population.
Acknowledgements
AJ Barrett is supported by the Intramural Branch of the National Heart, Lung and Blood Institute, NIH.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Batlevi CL, Matsuki E, Brentjens RJ, et al. Novel immunotherapies in lymphoid malignancies. Nat Rev Clin Oncol 2016;13:25-40. [Crossref] [PubMed]
- Klinger M, Benjamin J, Kischel R, et al. Harnessing T cells to fight cancer with BiTE® antibody constructs - past developments and future directions. Immunol Rev 2016;270:193-208. [Crossref] [PubMed]
- Geyer MB, Brentjens RJ. Current clinical applications of chimeric antigen receptor (CAR) modified T cells. Cytotherapy 2016;18:1393-409. [Crossref] [PubMed]
- May MB, Glode A. Blinatumomab: A novel, bispecific, T-cell engaging antibody. Am J Health Syst Pharm 2016;73:e6-e13. [Crossref] [PubMed]
- Dombret H, Gardin C. An update of current treatments for adult acute myeloid leukemia. Blood 2016;127:53-61. [Crossref] [PubMed]
- De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J 2016;6:e441. [Crossref] [PubMed]
- Finn LE, Foran JM. Are we curing more older adults with acute myeloid leukemia with allogeneic transplantation in CR1? Curr Opin Hematol 2016;23:95-101. [Crossref] [PubMed]
- van Besien K. Allogeneic transplantation for AML and MDS: GVL versus GVHD and disease recurrence. Hematology Am Soc Hematol Educ Program 2013;2013:56-62. [Crossref] [PubMed]
- Ishii K, Barrett AJ. Novel immunotherapeutic approaches for the treatment of acute leukemia (myeloid and lymphoblastic). Ther Adv Hematol 2016;7:17-39. [Crossref] [PubMed]
- Austin R, Smyth MJ, Lane SW. Harnessing the immune system in acute myeloid leukaemia. Crit Rev Oncol Hematol 2016;103:62-77. [Crossref] [PubMed]
- Goswami M, McGowan KS, Lu K, et al. A multigene array for measurable residual disease detection in AML patients undergoing SCT. Bone Marrow Transplant 2015;50:642-51. [Crossref] [PubMed]
- Loke J, Khan JN, Wilson JS, et al. Mylotarg has potent anti-leukaemic effect: a systematic review and meta-analysis of anti-CD33 antibody treatment in acute myeloid leukaemia. Ann Hematol 2015;94:361-73. [Crossref] [PubMed]
- Gill S, Tasian SK, Ruella M, et al. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood 2014;123:2343-54. [Crossref] [PubMed]
- Pizzitola I, Anjos-Afonso F, Rouault-Pierre K, et al. Chimeric antigen receptors against CD33/CD123 antigens efficiently target primary acute myeloid leukemia cells in vivo. Leukemia 2014;28:1596-605. [Crossref] [PubMed]
- Harrington KH, Gudgeon CJ, Laszlo GS, et al. The Broad Anti-AML Activity of the CD33/CD3 BiTE Antibody Construct, AMG 330, Is Impacted by Disease Stage and Risk. PLoS One 2015;10:e0135945. [Crossref] [PubMed]
- Testa U, Pelosi EA. CD 123 is a membrane biomarker and a therapeutic target in hematologic malignancies. Biomark Res 2014;2:4-11. [Crossref] [PubMed]
- Chichili GR, Huang L, Li H, et al. A CD3xCD123 bispecific DART for redirecting host T cells to myelogenous leukemia: preclinical activity and safety in nonhuman primates. Sci Transl Med 2015;7:289ra82. [Crossref] [PubMed]
- Al-Hussaini M, Rettig MP, Ritchey JK, et al. Targeting CD123 in acute myeloid leukemia using a T-cell-directed dual-affinity retargeting platform. Blood 2016;127:122-31. [Crossref] [PubMed]
- Johnson S, Burke S, Huang L, et al. Effector cell recruitment with novel Fv-based dual-affinity re-targeting protein leads to potent tumor cytolysis and in vivo B-cell depletion. J Mol Biol 2010;399:436-49. [Crossref] [PubMed]
- Rossi DL, Rossi EA, Cardillo TM, et al. A new class of bispecific antibodies to redirect T cells for cancer immunotherapy. MAbs 2014;6:381-91. [Crossref] [PubMed]
- Moore PA, Zhang W, Rainey GJ, et al. Application of dual affinity retargeting molecules to achieve optimal redirected T-cell killing of B-cell lymphoma. Blood 2011;117:4542-51. [Crossref] [PubMed]
- Sun Q, Woodcock JM, Rapoport A, et al. Monoclonal antibody 7G3 recognizes the N-terminal domain of the human interleukin-3 (IL-3) receptor alpha-chain and functions as a specific IL-3 receptor antagonist. Blood 1996;87:83-92. [PubMed]
- Vergez F, Green AS, Tamburini J, et al. High levels of CD34+CD38low/-CD123+ blasts are predictive of an adverse outcome in acute myeloid leukemia: a Groupe Ouest-Est des Leucemies Aigues et Maladies du Sang (GOELAMS) study. Haematologica 2011;96:1792-8. [Crossref] [PubMed]
- Frey NV, Porter DL. Cytokine release syndrome with novel therapeutics for acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program 2016;2016:567-72. [Crossref] [PubMed]
- Zhang Q, Hossain DM, Duttagupta P, et al. Serum-resistant CpG-STAT3 decoy for targeting survival and immune checkpoint signaling in acute myeloid leukemia. Blood 2016;127:1687-700. [Crossref] [PubMed]


