Therapeutic potential of targeting microRNAs to regulate cardiac fibrosis: miR-433 a new fibrotic player
Introduction
In response to stress, the heart undergoes cardiac remodelling to compensate for the increase in workload, and if left untreated can progress to heart failure and death. One of the key processes associated with pathological remodelling is the excessive extracellular matrix production by cardiac fibroblasts (i.e., cardiac fibrosis). Cardiac fibrosis reduces cardiac compliance, making the heart stiff, and this contributes to cardiac dysfunction and ultimately heart failure (1). At present, there is no therapy for cardiac fibrosis. Since the discovery of microRNAs (miRNAs) about 20 years ago, they have attracted much attention from numerous fields of biology and medicine, and are considered potential therapeutic targets. MicroRNAs are small (22 nucleotides), highly conserved non-coding RNA molecules (initially considered “junk”) that can regulate gene expression by binding to the 3’ untranslated region of its target mRNA (2). Numerous miRNAs are aberrantly expressed in settings of cardiovascular disease, and small molecule drugs that can modulate the expression of miRNAs have shown success in preclinical studies in cardiac stress settings (3).
miR-433: a new regulator of cardiac fibrosis
In a recent study published in the journal of Theranostics, Tao et al. described a novel role for miR-433 in regulating cardiac fibrosis via the transforming growth factor beta (TGFβ) and MAPK kinase (ERK/P38) pathways (4). To identify miRNAs which could potentially regulate cardiac fibrosis, the authors first performed a miRNA array in hearts of mice subjected to myocardial infarction (MI; ventricular tissue collected 21 days post-MI). Using a selection criteria of miRNA expression with a fold change of greater than 2.0 compared with sham (P<0.05), 26 miRNAs were identified to be dysregulated following MI. miR-433 was within the top four miRNAs upregulated following MI (6.35 fold versus sham) and represented a miRNA with an unknown role. Only miR-34b and miR-34c showed greater upregulation with MI but have previously been studied (5). miR-433 was also shown to be elevated in cardiac tissue of an independent mouse model associated with cardiac fibrosis (doxorubicin-induced cardiomyopathy) and in cardiac tissue from patients with dilated cardiomyopathy (4).
Fibroblast proliferation and myofibroblast differentiation are key events during cardiac fibrosis. Tao and colleagues showed that (I) miR-433 was enriched in cardiac fibroblasts in comparison to myocytes; and (II) forced expression of miR-433 in cultured cardiac fibroblasts increased fibroblast proliferation and myofibroblast differentiation; inhibition of miR-433 showed the opposite results (4). Next, the authors examined the therapeutic potential of inhibiting miR-433 in a cardiac disease model using a systemic or cardiac-specific approach. In the MI mouse model, both systemic inhibition of miR-433 (using a miR-433 antagomir—a 2' OME and 5' cholesterol modified miR-433 inhibitor) or cardiac specific inhibition [using an adeno-associated viral vector (AAV9) and miRNA sponge technology] attenuated the development of cardiac fibrosis and cardiac dysfunction. As cardiac fibrosis was not completely prevented, this result suggests the involvement of other pro-fibrotic miRNAs and/or signalling pathways. Numerous signalling pathways are known to be involved with the early activation of cardiac fibroblasts and pathological cardiac remodelling after an insult (1).
Two targets of miR-433 were identified (AZIN1 and JNK1) as contributing to the fibrotic properties of miR-433. AZIN1 had previously been shown to be a target of miR-433 and to regulate renal fibrosis (6), but the role of AZIN1 in regulating cardiac fibrosis was unknown. JNK1 was a predicted target of miR-433 and was also considered a candidate with the capacity to regulate cardiac fibrosis. Utilizing a variety of approaches (fibroblast cell culture, luciferase assays, siRNA, miR-433 agomiR/antagomiR), the authors present data to suggest that (I) AZIN1 and JNK1 are targets of miR-433 in cardiac fibroblasts, and regulate fibroblast proliferation and myofibroblast differentiation, (II) reduced AZIN1 expression activates TGFβ/Smad3 signalling in cardiac fibroblasts, (III) decreased levels of JNK increase the phosphorylation of ERK, P38 kinase and Smad3 and this contributes to fibroblast proliferation and the differentiation into myofibroblasts (IV) (summarized in Figure 1).
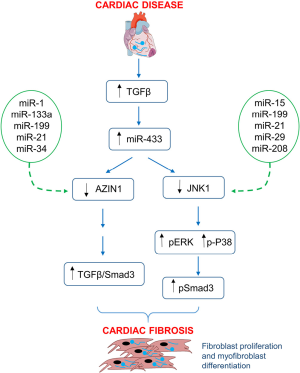
Other miRNAs implicated in regulating cardiac fibrosis
In the current study, of the 26 miRNAs identified to be dysregulated in a setting of MI, the top three miRNAs [based on fold-change; see Supp Table 2 of reference (4)] belong to the miR-34 family, while number six and eight on the list was miR-21a-5p and miR-154-5p, respectively. Each of these miRNAs, or their family members, have previously been shown to be elevated in hearts of cardiac stress mouse models associated with fibrosis (e.g., MI, pressure overload, dilated cardiomyopathy and atrial fibrillation), and targeting these miRNAs was effective in preventing or attenuating fibrosis in vivo (5,7-14). Other studies have identified numerous additional miRNAs dysregulated in cardiac fibrotic models. miRNAs implicated in regulating fibrosis based on in vivo studies [knockout models, transgenics, gene delivery (AAV/adenovirus) and miRNA oligonucleotide based (antimiR/antagomiR)] include miR-29, miR-15, miR-34a, miR-101, miR-132, miR-1, mR-133, miR-208a, miR-199b, miR-22 and miR-489 (15). These other pro-fibrotic miRNAs may act via similar or separate pathways to miR-433 in the heart. Tao and colleagues identified AZIN1 and JNK1 as key targets of miR-433 responsible for the pro-fibrotic effects. Of interest, AZIN1 and JNK1 are also predicted targets of other miRNAs implicated in regulating fibrosis (TargetScan version 7.1—AZIN1: miR-1, miR-133a, miR-199, miR-21, miR-34; JNK1: miR-15, miR-199, miR-21, miR-29, miR-208) (see Figure 1).
Unanswered questions, challenges and future directions
The studies described above highlight the potential of miRNA-based therapies to attenuate or protect against cardiac fibrosis in preclinical disease models. Most studies, to date, have examined cardiac fibrosis at study end point only i.e., fibrosis has not been examined serially within the same animal. Thus, the capacity of miRNA-based therapies to reverse established cardiac fibrosis remains largely unknown. Collagen turnover in the human heart is likely to be low (16,17). Consequently, any anti-fibrotic therapy for the human heart may need to be administered over a long time frame (6–12 months) before seeing any clear therapeutic benefit (18). In future studies it would be interesting to investigate whether the inhibition of miR-433 and other pro-fibrotic miRNAs is able to regress/reverse pre-existing fibrosis, as in most cases, patients are likely to first present at the clinic with established fibrosis.
The field of miRNA therapeutics is gaining momentum, as an inhibitor for miR-122 was deemed safe, well tolerated and reduced Hepatitis C viral RNA levels in a phase II clinical trial (19). A drawback of miRNA therapeutics in the heart is organ specificity as miRNA drugs are usually administered systemically and accumulate in the liver and kidney (3). To ensure sufficient uptake in the heart, higher doses are needed which may cause unwanted side effects. The majority of the miRNAs described in this commentary have widespread distribution. Thus, delivery approaches that selectively target the heart (and ideally fibroblasts) are likely to be required for miRNA-based drugs to enter the clinic for cardiac fibrotic disease. Additional complexities include the recognition that (I) miRNAs can be differentially regulated in the diseased heart of males and females (9), and (II) miRNA-based therapies can regulate the expression of secondary miRNAs via miRNA-transcription factor networks (20).
Despite these complexities and challenges, since the treatment of heart failure-associated fibrosis represents an unmet clinical need, identifying key dysregulated miRNAs responsible for cardiac fibrosis is considered important for improving our understanding of fibrotic pathways, and the development of novel therapies. The study by Tao and colleagues adds miR-433 as an important new regulator of cardiac fibrosis.
Acknowledgements
JR McMullen is a National Health and Medical Research Council Senior Research Fellow (1078985). BC Bernardo is supported by an Alice Baker and Eleanor Shaw Fellowship (Baker Foundation, Melbourne, Australia).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Travers JG, Kamal FA, Robbins J, et al. Cardiac Fibrosis: The Fibroblast Awakens. Circ Res 2016;118:1021-40. [Crossref] [PubMed]
- Bernardo BC, Charchar FJ, Lin RC, et al. A microRNA guide for clinicians and basic scientists: background and experimental techniques. Heart Lung Circ 2012;21:131-42. [Crossref] [PubMed]
- Bernardo BC, Ooi JY, Lin RC, et al. miRNA therapeutics: a new class of drugs with potential therapeutic applications in the heart. Future Med Chem 2015;7:1771-92. [Crossref] [PubMed]
- Tao L, Bei Y, Chen P, et al. Crucial Role of miR-433 in Regulating Cardiac Fibrosis. Theranostics 2016;6:2068-83.
- Bernardo BC, Gao XM, Winbanks CE, et al. Therapeutic inhibition of the miR-34 family attenuates pathological cardiac remodeling and improves heart function. Proc Natl Acad Sci U S A 2012;109:17615-20. [Crossref] [PubMed]
- Li R, Chung AC, Dong Y, et al. The microRNA miR-433 promotes renal fibrosis by amplifying the TGF-β/Smad3-Azin1 pathway. Kidney Int 2013;84:1129-44. [Crossref] [PubMed]
- Bernardo BC, Gao XM, Tham YK, et al. Silencing of miR-34a attenuates cardiac dysfunction in a setting of moderate, but not severe, hypertrophic cardiomyopathy. PLoS One 2014;9:e90337. [Crossref] [PubMed]
- Thum T, Gross C, Fiedler J, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008;456:980-4. [Crossref] [PubMed]
- Bernardo BC, Ooi JY, Matsumoto A, et al. Sex differences in response to miRNA-34a therapy in mouse models of cardiac disease: identification of sex-, disease- and treatment-regulated miRNAs. J Physiol 2016;594:5959-74. [Crossref] [PubMed]
- Sun LY, Bie ZD, Zhang CH, et al. MiR-154 directly suppresses DKK2 to activate Wnt signaling pathway and enhance activation of cardiac fibroblasts. Cell Biol Int 2016;40:1271-9. [Crossref] [PubMed]
- Yang Y, Cheng HW, Qiu Y, et al. MicroRNA-34a Plays a Key Role in Cardiac Repair and Regeneration Following Myocardial Infarction. Circ Res 2015;117:450-9. [Crossref] [PubMed]
- Boon RA, Iekushi K, Lechner S, et al. MicroRNA-34a regulates cardiac ageing and function. Nature 2013;495:107-10. [Crossref] [PubMed]
- Bernardo BC, Nguyen SS, Gao XM, et al. Inhibition of miR-154 Protects Against Cardiac Dysfunction and Fibrosis in a Mouse Model of Pressure Overload. Sci Rep 2016;6:22442. [Crossref] [PubMed]
- Lin RC, Weeks KL, Gao XM, et al. PI3K(p110 alpha) protects against myocardial infarction-induced heart failure: identification of PI3K-regulated miRNA and mRNA. Arterioscler Thromb Vasc Biol 2010;30:724-32. [Crossref] [PubMed]
- Creemers EE, van Rooij E. Function and Therapeutic Potential of Noncoding RNAs in Cardiac Fibrosis. Circ Res 2016;118:108-18. [Crossref] [PubMed]
- Zile MR, Baicu CF, Ikonomidis JS, et al. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation 2015;131:1247-59. [Crossref] [PubMed]
- Ellims AH, Taylor AJ, Mariani JA, et al. Evaluating the utility of circulating biomarkers of collagen synthesis in hypertrophic cardiomyopathy. Circ Heart Fail 2014;7:271-8. [Crossref] [PubMed]
- McLellan AJ, Schlaich MP, Taylor AJ, et al. Reverse cardiac remodeling after renal denervation: Atrial electrophysiologic and structural changes associated with blood pressure lowering. Heart Rhythm 2015;12:982-90. [Crossref] [PubMed]
- Janssen HL, Reesink HW, Lawitz EJ, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med 2013;368:1685-94. [Crossref] [PubMed]
- Ooi JY, Bernardo BC, Singla S, et al. Identification of miR-34 regulatory networks in settings of disease and antimiR-therapy: Implications for treating cardiac pathology and other diseases. RNA Biol 2016. [Epub ahead of print]. [Crossref] [PubMed]


