Uncovering the influence of the FGFR1 pathway on glioblastoma radiosensitivity
Gliomas are the most frequently occurring brain tumour, of which glioblastomas (WHO grade IV gliomas) are the most common subtype and carry a particularly poor prognosis, leading to 5-year survival rates of just 9.8% (1,2). Radiation is an effective treatment modality although dose escalation is limited by the narrow therapeutic index. Consequently, there is a clear and unmet clinical need for effective radiosensitisation strategies for glioblastoma which enhance anti-tumour efficacy without increasing dose-limiting normal tissue toxicity. The paucity of effective clinically validated radiosensitisers further highlights the need for novel therapeutic targets for radiosensitisation of glioblastoma.
Fibroblast growth factor receptor (FGFR) aberrations, in the form of mutations, amplifications or translocations are commonly found in breast and non-small cell lung cancer (3,4). In glioblastoma, FGFR mutations are found in 3% of cases and are the result of fusions between the tyrosine kinase coding domain of the FGFR gene with the transforming acidic coiled-coil (TACC) domain of the evolutionary conserved TACC1 and TACC3 genes involved in mitotic spindle localisation (5,6). FGFR1 has been shown to have a role in resistance to cytotoxic and hormonal therapies in a variety of tumour types (7,8). FGFR1 expression levels have been shown to be a poor predictive marker of overall survival and time to progression in patients treated with chemo-radiotherapy in glioblastoma (9). The predictive role of FGFR1 may be due to modulation of the tumour microenvironment and angiogenic response which may in turn influence tumour radiosensitivity. Cohen-Jonathan-Moyal and colleagues have investigated extensively the role of FGFR signalling in contributing to radiosensitivity (10) and have also shown that the pan-FGFR inhibitor, SSR128129E, increases radiosensitivity in glioblastoma both in vitro and in vivo (11). Most recently, they demonstrated for the first time that the FGFR1 pathway is implicated in the in vitro and in vivo radioresistance of glioblastoma (12). In their study they made use of human glioblastoma cell lines, U87 and LN18 which are known to express FGFR1, in combination with shRNA and siRNA to silence FGFR1 in both in vitro and in vivo settings. Using clonogenic assays to measure the surviving fraction after 2 Gy irradiation (SF2), it was shown that FGFR1 inhibition increased the radiosensitivity of both cell lines (12). Further investigation demonstrated that the increase in radiation-induced death was associated with mitotic cell death as evidenced by an increased percentage of giant multinucleated cells and increased centrosome overduplication following silencing of FGFR1 (12).
Next, Gouazé-Andersson et al., investigated the mechanism by which FGFR1 mediates a radioprotective effect and determined that this was at least in part dependent on phospholipase C gamma (PLCγ). The SF2 of cells deficient in PLCγ (using siRNA) decreased by 28% in U87 cells and 33.9% in LN18 cells compared to control lines. Likewise, the percentage of giant multinucleated cells and centrosome overduplication increased upon depletion of PLCγ (12). Together, these results strongly suggest that FGFR1-mediated radioresistance is dependent, at least partly, on PLCγ signalling. Further mechanistic insight could be achieved by assessing radiosensitivity in FGFR1 null cell lines following PLCγ inhibition which may help to clarify the relative contribution of PLCγ signalling to FGFR1-mediated radioresistance (Figure 1).
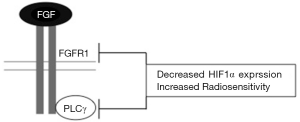
Glioblastomas are characterised by areas of hypoxia and necrosis which drives hypoxia-mediated tumour growth (13). HIF1 signalling is the primary orchestrator of the cellular response to hypoxia (14). HIF1 is a heterodimeric transcription factor consisting of α and β subunits which increase expression of genes implicated in gliomagenesis, angiogenesis and invasion (13). In a recent meta-analysis, high HIF1α expression was confirmed as a poor prognostic marker in glioblastoma (15). Most work to date has investigated the role of HIF1α signalling in the context of hypoxia. However, HIF1α can also be expressed and regulated under conditions of normal oxygen levels through the activity of oncogenes, growth factors and free radicals (16). For example, activation of Epidermal Growth Factor Receptor (EGFR) signalling has been shown to increase HIF1α expression via the phosphatidylinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/PKB/mTOR) pathway in thyroid cancer (17).
HIF1α expression levels vary between different glioblastoma cell lines in normoxia. Previous work on U251MG and U343MG human glioblastoma cell lines have shown low levels of HIF1α (18) whereas the cell lines, U87 and LN18, used in this study show high levels of HIF1α in normoxia (12). The significance of these varying expression levels on tumour progression and therapy response remains unclear. In this study it was demonstrated that silencing FGFR1 and PLCγ decreased HIF1α expression in normoxia. Specifically, in the U87 cell line, HIF1α expression decreased 11.1 fold and 2.38 fold upon depletion of FGFR1 and PLCγ respectively (12). Reductions in HIF1α levels were also seen in the LN18 cell line upon silencing FGFR1 and PLCγ. Loss of PLCγ expression produced a greater (11.1 fold) decrease in HIF1α expression, suggesting that additional factors may be involved in the FGFR1 and PLCγ mediated regulation of HIF1α expression (12). These are the first published data suggesting an interconnecting role of FGFR1 and PLCγ signalling in mediating radioresistance in glioblastoma. Further exciting insight may be gained by contrasting the effect of FGFR1 and PLCγ silencing in the context of hypoxia and normoxia.
FGFR1-silenced U87 cells were then used in xenograft studies and showed that FGFR1 inhibition alone had no impact on the rate of tumour growth. However, increased radiosensitivity was seen when FGFR1 inhibition was combined with a single 5 Gy fraction of irradiation (12). As the commonly used radiation schedules for glioblastoma involve fraction doses of 1.8–5 Gy, this combination of FGFR1 depletion and irradiation suggests a feasible and effective radiosensitisation strategy. A similar approach was not taken in vivo combining knockdown of PLCγ and irradiation, although the prediction is that this would also increase radiosensitivity. In addition, it would be interesting to extend these studies to evaluate the impact of varying fraction sizes and fractionation schedules to reflect the spectrum of radiation dosing being used in clinical practice.
In support of their findings in vitro, when the FGFR1-silenced xenograft tumours were assessed using immunohistochemistry for HIF1α expression, a reduction was seen in both small (100 mm3) and large (3,000 mm3) tumours (12). It would be reasonable to expect and amenable to formal demonstration that hypoxic regions exist in these tumours. These data are consistent with previous reports showing that a pan-FGFR inhibitor reduces HIF1α expression levels in hypoxia (11). However, despite the decreased HIF1α expression in the FGFR1 depleted tumours there was no apparent change in the density or morphology of intra-tumoural blood vessels which suggests HIF1α levels remained sufficient to promote angiogenesis (12). As HIF1α has numerous roles including the regulation of cell proliferation, glucose metabolism and migration, it is possible that the FGFR1-mediated loss of HIF1α expression may impact other aspects of HIF biology. Of particular interest is the role of HIF1α expression in regulating radiosensitivity. Previous studies suggest HIF1 may increase radiosensitivity by maintaining glucose metabolism and ATP production in murine mammary and human colorectal xenograft models (19). On the other hand, pre-clinical evidence has also shown that combining radiation with HIF1α inhibition can overcome radioresistance in malignant gliomas (18). The difference in radiosensitivity outcomes may be related to the diverse roles of HIF1α signalling in pathways known to influence radiosensitivity such as cell proliferation (19) and the inherent variation seen between varying tumour models and experimental technique.
Currently, there are both selective and non-selective FGFR tyrosine kinase inhibitors (TKI) in phase I and II clinical development. Of the inhibitors available the use of monoclonal antibodies against FGFR offer the most selectivity, however these studies are in their infancy (20). Non-selective FGFR inhibitors, such as dovitinib, have shown modest bioactivity against FGFR signalling in metastatic breast cancer (21). Tolerability was low due to off-target VEGF inhibition by these non-selective FGFR inhibitors, resulting in hypertension and proteinuria (22). More selective and potent FGFR TKIs, for example BGJ398, are being investigated in a range of solid tumours with the hope of an improved toxicity profile (22). There are no current studies evaluating FGFR inhibition in glioblastoma as either monotherapy or in combination with radiotherapy. Therefore, the opportunity exists to combine these preclinical data with the available and developing clinical tools to translate preclinical mechanistic insight to a clinical setting for a malignancy of significant unmet need.
Deciphering the mechanisms of radioresistance in glioblastoma could significantly improve our therapeutic outcomes in this poor prognostic disease. In this study, there is evidence that FGFR1 signalling contributes to the radioresistant phenotype. FGFR1 may also be implicated in normoxia-dependent regulation of HIF1α expression. Many mechanistic questions remain unanswered which must be addressed to develop the therapeutic avenues essential in improving the clinical efficacy of radiation in glioblastoma.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Tanaka S, Louis DN, Curry WT, et al. Diagnostic and therapeutic avenues for glioblastoma: no longer a dead end? Nat Rev Clin Oncol 2013;10:14-26. [Crossref] [PubMed]
- Borovski T, Beke P, van Tellingen O, et al. Therapy-resistant tumor microvascular endothelial cells contribute to treatment failure in glioblastoma multiforme. Oncogene 2013;32:1539-48. [Crossref] [PubMed]
- Theillet C, Adelaide J, Louason G, et al. FGFRI and PLAT genes and DNA amplification at 8p12 in breast and ovarian cancers. Genes Chromosomes Cancer 1993;7:219-26. [Crossref] [PubMed]
- Weiss J, Sos ML, Seidel D, et al. Frequent and focal FGFR1 amplification associates with therapeutically tractable FGFR1 dependency in squamous cell lung cancer. Sci Transl Med 2010;2:62ra93. [Crossref] [PubMed]
- Peset I, Vernos I. The TACC proteins: TACC-ling microtubule dynamics and centrosome function. Trends Cell Biol 2008;18:379-88. [Crossref] [PubMed]
- Singh D, Chan JM, Zoppoli P, et al. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science 2012;337:1231-5. [Crossref] [PubMed]
- Turner N, Pearson A, Sharpe R, et al. FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res 2010;70:2085-94. [Crossref] [PubMed]
- Fernanda Amary M, Ye H, Berisha F, Khatri B, et al. Fibroblastic growth factor receptor 1 amplification in osteosarcoma is associated with poor response to neo-adjuvant chemotherapy. Cancer Med 2014;3:980-7. [Crossref] [PubMed]
- Ducassou A, Uro-Coste E, Verrelle P, et al. αvβ3 Integrin and Fibroblast growth factor receptor 1 (FGFR1): Prognostic factors in a phase I-II clinical trial associating continuous administration of Tipifarnib with radiotherapy for patients with newly diagnosed glioblastoma. Eur J Cancer 2013;49:2161-9. [Crossref] [PubMed]
- Fuks Z, Persaud RS, Alfieri A, et al. Basic fibroblast growth factor protects endothelial cells against radiation-induced programmed cell death in vitro and in vivo. Cancer Res 1994;54:2582-90. [PubMed]
- Ader I, Delmas C, Skuli N, et al. Preclinical evidence that SSR128129E--a novel small-molecule multi-fibroblast growth factor receptor blocker--radiosensitises human glioblastoma. Eur J Cancer 2014;50:2351-9. [Crossref] [PubMed]
- Gouazé-Andersson V, Delmas C, Taurand M, et al. FGFR1 Induces Glioblastoma Radioresistance through the PLCγ/Hif1α Pathway. Cancer Res 2016;76:3036-44. [Crossref] [PubMed]
- Kaur B, Khwaja FW, Severson EA, et al. Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro Oncol 2005;7:134-53. [Crossref] [PubMed]
- Lee JW, Bae SH, Jeong JW, et al. Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions. Exp Mol Med 2004;36:1-12. [Crossref] [PubMed]
- Liu Q, Cao P. Clinical and prognostic significance of HIF-1α in glioma patients: a meta-analysis. Int J Clin Exp Med 2015;8:22073-83. [PubMed]
- Ranasinghe WK, Baldwin GS, Shulkes A, et al. Normoxic regulation of HIF-1α in prostate cancer. Nat Rev Urol 2014;11:419. [Crossref] [PubMed]
- Clarke K, Smith K, Gullick WJ, et al. Mutant epidermal growth factor receptor enhances induction of vascular endothelial growth factor by hypoxia and insulin-like growth factor-1 via a PI3 kinase dependent pathway. Br J Cancer 2001;84:1322-9. [Crossref] [PubMed]
- Kessler J, Hahnel A, Wichmann H, et al. HIF-1α inhibition by siRNA or chetomin in human malignant glioma cells: effects on hypoxic radioresistance and monitoring via CA9 expression. BMC Cancer 2010;10:605. [Crossref] [PubMed]
- Moeller BJ, Dreher MR, Rabbani ZN, et al. Pleiotropic effects of HIF-1 blockade on tumor radiosensitivity. Cancer Cell 2005;8:99-110. [Crossref] [PubMed]
- Dieci MV, Arnedos M, Andre F, et al. Fibroblast growth factor receptor inhibitors as a cancer treatment: from a biologic rationale to medical perspectives. Cancer Discov 2013;3:264-79. [Crossref] [PubMed]
- André F, Bachelot T, Campone M, et al. Targeting FGFR with dovitinib (TKI258): preclinical and clinical data in breast cancer. Clin Cancer Res 2013;19:3693-702. [Crossref] [PubMed]
- Lewin J, Siu LL. Development of Fibroblast Growth Factor Receptor Inhibitors: Kissing Frogs to Find a Prince? J Clin Oncol 2015;33:3372-4. [Crossref] [PubMed]


