Anticoagulant independent mechanical heart valves: viable now or still a distant holy grail
Introduction
Valvular heart disease remains a significant problem for both developing as well as for developed societies and continues to be the subject of intense focus for public health organizations, researchers, and governments. Over the past 65 years since the first orthotopic valve replacement, the predominance of valvular heart disease has changed in industrialized countries from an infectious and preventable etiology, towards a degenerative one, due in large part, to population aging along with preventative containment. Conversely, rheumatic fever remains the principal cause of acquired heart disease in developing countries (1,2). The World Health Organization in collaboration with The World Heart Federation reported that “the global burden of disease caused by Rheumatic Fever and Rheumatic Heart Disease (RHD) currently falls disproportionately on children and young adults living in low-income countries and is responsible for about 233,000 deaths annually.” It is estimated that at least 15.6 million people are currently affected by RHD, and a significant number of them will require repeated hospitalization and be candidates for heart surgery in the next 5 to 20 years (3). Thus, the epidemiology, clinical presentation and treatment options for valvular heart disease are substantially more challenging for these countries than those for North America, the European Union and other industrialized regions. Furthermore, over the past 65-year interval, important changes have occurred in valve pathology and patient characteristics, which have stimulated new forms of treatment, advances in surgical techniques and development of new valvular prostheses.
Presently, the choice of valve prosthesis is either tissue-based (BHV) or mechanical (MHV). The discussion continues regarding which type is preferential in a given patient population according to age, valve pathology, and anatomic location. Central to the discussion is the ongoing dilemma regarding trade-offs on durability, need for oral anticoagulants, the incidence of complications related to these medications and suboptimal hemodynamics as well as treatment and device cost (4-7).
Largely due to the impression that recipients will be free of chronic warfarin based anticoagulation therapy, tissue valves have become increasingly popular, with a 43.6% increase over ten years in the U.S. by one estimate (Brown et al.) (8) and 12.4% over two years in the E.U. by another (Dunning et al.) (9). The adoption of catheter deployed valves has accelerated this trend, this despite data showing that 20–35% of tissue valve recipients require anti-coagulation for non-valve as well as valve-related issues, including, pre-existing history of atrial fibrillation or stroke, interaction between the frequently retained native calcified aortic valves and the prostheses, and differences in valve prosthesis geometry within 5 years of the original surgery (10). Paradoxically, this has created support for earlier and longer warfarin therapy, as proposed by Mérie and associates (11) who challenge present anticoagulation guidelines based on their study of 4075 patients after bioprosthetic aortic valve replacement. Over a period of 10 years, (a median follow-up of 6.5 years) they demonstrated that discontinuation of warfarin therapy within the first 3 months after surgery is associated with a statistically significant increase in the risk of stroke and other thromboembolic complications; if the medication is discontinued within 90 to 179 days after surgery, they find a significant increase in cardiovascular death and in comparison, if warfarin therapy is maintained for up to 730 days they find a significantly lower mortality.
This, together with the recently published work by Makkar et al. (12) describing reduced aortic-valve leaflet motion in patients after transcatheter valve implantation (TAVI) or surgically placed bioprosthetic valves, as well as by Egbe et al. (13) asserting that bioprosthetic valve thrombosis is not uncommon and can occur several years after surgery, has added to the discussion. These alarming observations raise concerns that patients undergoing either TAVI or surgical aortic valve replacement (SAVR) with bioprosthesis, could be at higher risk for leaflet thrombosis and consequent embolic stroke than previously assumed by patients and their physicians.
Unresolved are questions regarding antithrombotic therapy in the setting of TAVI including, type and dose of medication used during a procedure and type, dose and duration of treatment post procedure (10). Ghanem and associates state (14) “…assume that future studies will concentrate on synergistic effects of antiplatelet therapy (APT) and novel oral anticoagulants (NOACs) in adapted dosage after TAVI, to address the underlying complex pathophysiological mechanisms of subacute stroke.” The controversy has not been resolved by the existing guidelines (15,16), as numerous publications report better long-term survival for mechanical valve recipients compared with those with tissue valves (9,17-24). Difficulties in patient recruitment notwithstanding, systematic, randomized studies of this phenomenon are necessary in order to clarify the mechanism, assess clinical consequences and satisfy ethical obligations to provide complete and accurate information to patients regarding risks and benefits of a biological or a mechanical prosthesis. The challenges of oral anticoagulation and limited durability are major concerns in developing societies, mostly due to the early age of onset, rapid progression of the disease and lack of ability for monitoring warfarin based anticoagulation. The convenience of home monitoring as well as safety of lower dose warfarin for specific aortic valve models remains impractical for developing countries. Consequently, while patients from remote areas lack access to anticoagulation monitoring facilities and associated therapies, it is mainly limited tissue valve durability that makes them an unattractive choice in regions where potential recipients might be as young as 20 years of age.
Less than deserved consideration has been given to this well documented data, perhaps due to frequent reports on TAVI successes and advances in instrumentation, intended to treat a high-risk, elderly, (average age 80 to 85 years), population of patients with aortic valve disease not deemed suitable for surgical valve replacement. Until recently, the majority of these patients have been followed for a short or midterm period (2 to 5 years). The early results have expanded the use of tissue valves in general, and TAVI in particular, to a younger and lower risk population. More recently, reports with longer follow-up in younger patients have appeared calling for caution (17,18). According to Dvir et al., the curve of degeneration in transcatheter valves (TCV) exhibits a steep acceleration at 5 years and exceeds 50% at eight (25). These researchers state: “Physicians must be mindful of limitations of the bioprosthesis they implant and whether these valves can be safely and effectively treated by a transcatheter approach (Valve-in-Valve), if these valves fail years later”.
An important and unintended consequence of the tissue valve trend is that mechanical valve innovation by major manufacturers has virtually ceased. This has occurred despite questions regarding tissue valve durability, suboptimal hemodynamics in smaller sizes, and a significant requirement for chronic anticoagulation therapy by recipients. Although there has been steady improvement in mechanical designs over the years, the most recent commercially successful valve, the On-X valve (On-X Life Technologies Inc®, Austin, Texas, USA) is over 20 years old. This prosthesis incorporated advances in carbon materials technology and other improvements related to valve functionality and production. Based upon the “‘Prospective Randomized’ On-X Anti-Coagulation Trial” (PROACT), the FDA approved warfarin management of selected aortic valve patients at INR levels between 1.5 and 2.0. However, with a mean INR of 1.9 some adverse events were noted in patients managed near the 1.5 lower limit (26). Other investigators have proposed home monitoring and lower INR targets in patients with mechanical heart valves (27). While encouraging, this is at best only a small step forward and does not alter the fundamental dilemma in mechanical versus biologic valve decision-making. A major advance in mechanical valve performance is required that will resolve this global problem (28).
What are the optimal valve prosthesis requirements?
Over a half century has passed since Dr. D. E. Harken in 1960 proposed his “Ten Commandments” of the ideal prosthetic mechanical heart valve substitute (29). In 1986, he reported to the World Congress on Heart Valve Replacement that all of his requirements continued to be “often quoted, but not completely met” (30) Table 1.
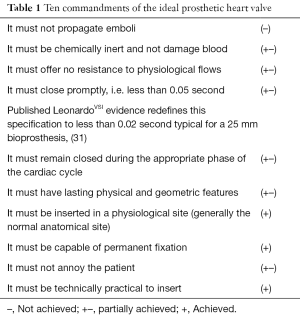
Full table
A proposal
The elusive “Holy Grail” for cardiac valve replacement continues to be a prosthesis that does not require anticoagulation, has excellent hemodynamic performance and is durable throughout the projected lifetime of all possible recipients. With improved technology together with knowledge and experience gained over the last quarter century, the time is overdue for development and introduction of a valve capable of achieving these goals and this is best accomplished with a mechanical prosthesis.
New prospects for success
In 2001 a 20-year retrospective study at Cedars-Sinai Medical Center of 2,533 valve recipients 18 years or older found no significant difference in survival or complication rates between patients with mechanical or bioprosthetic valves (32). These findings renewed the interest by researchers to explore new valve materials and designs (33). One result was development of more biostable polymers including new generations of polyurethanes and several new tri-leaflet valve designs, as well as an understanding of their durability related to leaflet stress concentration. Unfortunately, due in part to as yet unresolved complexity in manufacturing of this class of devices, this work on going for well over half a century has failed to produce acceptable polymeric prostheses with sufficient durability for human implantation and their only useful clinical application has been in heart assist devices (33,34).
A review of mechanical valve design history, based on tools and testing equipment available at the time, reveals well recognized problems and accepted limitations. For decades the focus was on forward flow hemodynamics, effective orifice areas, regurgitation, minimizing regions of stasis within the valve, reducing turbulence and decreasing cavitation to minimize common complications (35). These issues are certainly important and merit consideration for further improvement (36,37). More recently, however, the utilization of new, improved imaging technology has revealed other valve related motion and flow characteristics that need to be factored into valve design, in order to reduce blood damage and thrombosis. A recent concept is the presence of supra-physiologic pressure and flow transients with associated blood damaging high fluid shear at or immediately before valve closure observed with mechanical valves, but not with tissue valves. This can result in negative pressure spikes of up to 1,400 mm Hg as reported by Dexter et al. after implantation in animals, of a bileaflet valve in the mitral position (38). Scotten and Siegel confirmed this revelation using a simple opto-electronic methodology to measure occluder motion monitored by light passage through functioning test valves imaged on a silicon photodiode (31). This novel Fluid Velocity Assessment by Light Transmission (FVALT) system provides a spatial average of forward and retrograde flow velocities in the immediate valve proximity to screen for relative thrombogenic potential (39-41). This equipment can resolve regional flow velocities up to 200 meters per second (M/s), milliseconds prior to or at full valve closure; it has at an order of magnitude greater velocity measurement capability and far greater resolution compared to that of the most commonly used clinical diagnostic methods such as angiography and transthoracic, or transesophageal echocardiography. A state of the art Siemens SC 2000 echocardiographic ultrasound machine will measure velocity at a maximum of 14 M/s, but the most rapid velocity ever seen in clinical medicine is in the range of 5–5.5 M/s in the most severe aortic stenosis. These clinical measurements are made with the technician seeking the most rapid velocity “envelope” which is sinusoidal in form, encompassing the entire systolic or diastole phase. Within this envelope are a variety of velocities in which the highest determines the waveform configuration and the derived flow data. For instance with presently used clinical technology, a flow transient with a few milliseconds duration in contrast to a cardiac cycle length of 400–500 milliseconds (heart rate: 120–150 beats per minute), cannot be differentiated from an artifact (even if the velocity could be measured).
Additionally, Scotten and Siegel (39,40) have reported high amplitude, short duration regional backflow velocities (RBVs) across MHVs in vitro, that are considered a primary cause of shear rate damage to cells and other blood components. Considering that thrombus and/or embolization can be primarily induced by these clinically invisible RBVs, these findings implicate a possible way to evaluate potential anticoagulant independent valve prototypes in vitro. In turn, reducing in vitro detectable RBVs should help optimize prototype MHVs relative to tissue valve controls and contribute to development of future prototype valve designs that lower potential for thrombus formation/embolization (39-41).
In addition, over the last 20 years, the fields of computer-aided design and computational fluid dynamics have become more sophisticated and effective.
New valve bench testing equipment such as the prior mentioned FVALT provides a previously unavailable means to quantify localized blood flow between the valve housing and the occluder (s) throughout the full cardiac cycle. Other examples of advanced technology are microparticle image velocimetry to quantitatively measure flow behavior in small domains in the nanometer scale (42) and the Lattice-Boltzmann method employed successfully for fluid flow modeling including higher Reynolds numbers (43). Recent studies relating fluid shear to valve pro-thrombotic potential have identified crucial differences in mechanical and tissue valve closing responses (28). Another example of innovative new technology is the use of time resolved 3D MRI and 4D velocity encoded CMR for in vitro and in vivo flow characteristics studies (44-46). Manufacturing technologies have also advanced and are better able to effectively produce more complicated valve component geometry (47,48).
In conclusion, considering the entire spectrum of enhanced capabilities that have provided greater understanding of the relationship between flow characteristics, valve geometry, dynamic behavior and valve closure, these new data sources hold the promise of providing critical new insight into the possible causes of thromboembolism that can bring an anticoagulant independent mechanical valve within reach. Prompt and vigorous efforts to achieve this longstanding objective are called for.
Acknowledgements
The authors are grateful to the following for their generous contributions to this manuscript: Prediman Krishan Shah, MD, MACC, George E, Chaux, MD, and Robert W. Emery, MD.
Footnote
Conflicts of Interest: AC, RJG, JCS, MRE, are founding members of Lakeway Innovations LLC. The purpose of the company is to engage in design, development, and testing of medical devices, and technical consulting services. The stock they own in the company has no commercial value. Lakeway innovations LLC is a private start up company not listed in any exchange. The other authors have no conflicts of interest to declare.
References
- Iung B, Vahanian A. Epidemiology of valvular heart disease in the adult. Nature Reviews Cardiology 2011;8:162-172. [Crossref] [PubMed]
- Iung B, Vahanian A. Epidemiology of Acquired Valvular Heart Disease. Can J Cardiol 2014;30:962-970. [Crossref] [PubMed]
- Global Atlas on Cardiovascular Disease Prevention and Control. Mendis S, Puska P, Norrving B editors. World Health Organization (in collaboration with the World Heart Federation and World Stroke Organization), Geneva 2011.
- Schelbert EB, Vaughan-Sarrazin MS, Welke KF, et al. Valve type and long-term outcomes after aortic valve replacement in older patients. Heart 2008;94:1181-88. [Crossref] [PubMed]
- De Santo LS, Romano G, Della Corte A, et al. Mechanical aortic valve replacement in young women planning on pregnancy. J Am Coll Cardiol 2012;59:1110-5. [Crossref] [PubMed]
- Marijon E, Mirabel M, Celermajer D, et al. Rheumatic Heart Disease Seminar. Lancet 2012;379:953-64. [Crossref] [PubMed]
- Yoganathan AP, Fogel M, Gamble S, et al. A new paradigm for obtaining marketing approval for pediatric-sized prosthetic heart valves. J Thorac Cardiovasc Surg 2013;146:879-86. [Crossref] [PubMed]
- Brown JM, O'Brien SM, Wu C, et al. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg 2009;137:82-90. [Crossref] [PubMed]
- Dunning J, Gao H, Chambers J, et al. Aortic valve surgery: marked increases in volume and significant decreases in mechanical valve use--an analysis of 41,227 patients over 5 years from the Society for Cardiothoracic Surgery in Great Britain and Ireland National database. J Thorac Cardiovasc Surg 2011;142:776-782.e3. [Crossref] [PubMed]
- Rodés-Cabau J, Dauerman HL, Cohen MG, et al. Antithrombotic treatment in transcatheter aortic valve implantation. J Am Coll Cardiol 2013;62:2349-59. [Crossref] [PubMed]
- Mérie C, Køber L, Skov Olsen P, et al. Association of warfarin therapy duration after bioprosthetic aortic valve replacement with risk of mortality, thromboembolic complications, and bleeding. JAMA 2012;308:2118-25. [Crossref] [PubMed]
- Makkar RR, Fontana G, Jilaihawi H, et al. Possible subclinical leaflet thrombosis in bioprosthetic aortic valves. N Engl J Med 2015;373:2015-24. [Crossref] [PubMed]
- Egbe AC, Pislaru SV, Pellikaka PA, et al. Bioprosthetic valve thrombosis versus structural failure. Clinical and echocardiographic predictors. J Am Coll Cardiol 2015;66:2285-94. [Crossref] [PubMed]
- Ghanem A, Naderi AS, Frerker C, et al. Mechanisms And Prevention Of TAVI-Related Cerebrovascular Events. Curr Pharm Des 2016;22:1879-87. [Crossref] [PubMed]
- Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451-96. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:e521-643. [Crossref] [PubMed]
- Glaser N, Jackson V, Holzmann MJ, et al. Aortic valve replacement with mechanical vs. biological prostheses in patients aged 50-69 years. Eur Heart J 2016;37:2658-67. [Crossref] [PubMed]
- Head SJ, Kappetein AP. Aortic valve replacement in younger adults: a biological valve is not the logical choice. Eur Heart J 2016;37:2668-70. [Crossref] [PubMed]
- Banbury MK, Cosgrove DM, White JA, et al. Age and valve size effect on the long-term durability of the Carpentier-Edwards aortic pericardial bioprosthesis. Ann Thorac Surg 2001;72:753-7. [Crossref] [PubMed]
- Kaneko T, Aranki S, Javed Q, et al. Mechanical versus bioprosthetic mitral valve replacement in patients <65 years old. J Thorac Cardiovasc Surg 2014;147:117-26. [Crossref] [PubMed]
- Stassano P, Di Tommaso L, Monaco M, et al. Aortic valve replacement: a prospective randomized evaluation of mechanical versus biological valves in patients ages 55 to 70 years. J Am Coll Cardiol 2009;54:1862-8. [Crossref] [PubMed]
- Brown ML, Schaff HV, Lahr BD, et al. Aortic valve replacement in patients aged 50 to 70 years: Improved outcome with mechanical versus biologic prostheses. J Thorac Cardiovasc Surg 2008;135:878-84. [Crossref] [PubMed]
- Suri RM, Schaff HV. Selection of Aortic Valve Prostheses: Contemporary reappraisal of mechanical versus biologic valve substitutes. Circulation 2013;128:1372-80. [Crossref] [PubMed]
- Mohr F, Hamm CW, Welz A. Transcatheter Aortic-Valve Replacement in Clinical Practice. N Engl J Med 2016;374:1690-1. [Crossref] [PubMed]
- Dvir D, On behalf of coauthors: Eltchaninoff H, Ye J, Kan A, et al. First look at long-term durability of transcatheter heart valves: Assessment of valve function up to 10-years after implantation. euroPCR 2016.
- Puskas J, Gerdisch M, Nichols D, et al. Reduced anticoagulation after mechanical aortic valve replacement: interim results from the prospective randomized on-X valve anticoagulation clinical trial randomized Food and Drug Administration investigational device exemption trial. J Thorac Cardiovasc Surg 2014;147:1202-10. [Crossref] [PubMed]
- Emery RW, Emery AM, Raikar GV, Shake JG. Anticoagulation for Mechanical Heart Valves: a role for patient based therapy. J Thromb Thrombolysis 2008;25:18-25. [Crossref] [PubMed]
- Marom G, Bluestein D. Lagrangian methods for blood damage estimation in cardiovascular devices--How numerical implementation affects the results. Expert Rev Med Devices 2016;13:113-22. [Crossref] [PubMed]
- Harken DE, Soroff HS, Taylor WJ, et al. Partial and complete prostheses in aortic insufficiency. J Thorac Cardiovasc Surg 1960;40:744-62. [PubMed]
- Harken DE. Heart valves: ten commandments and still counting. Ann Thorac Surg 1989;48:S18-9. [Crossref] [PubMed]
- Scotten LN, Siegel R. Importance of shear in prosthetic valve closure dynamics. J Heart Valve Dis 2011;20:664-72. [PubMed]
- Khan SS, Trento A, DeRobertis M, et al. Twenty-year comparison of tissue and mechanical valve replacement. J Thorac Cardiovasc Surg 2001;122:257-69. [Crossref] [PubMed]
- Gharaie SH, Morsi Y. A novel design of a polymeric aortic valve. Int J Artif Organs 2015;38:259-70. [Crossref] [PubMed]
- Bezuidenhout D, Williams DF, Zilla P. Polymeric heart valves for surgical implantation, catheter-based technologies and heart assist devices. Biomaterials 2015;36:6-25. [Crossref] [PubMed]
- Yoganathan AP, Chaux A, Gray RJ, et al. Bileaflet, tiling disc and porcine substitutes: In vitro hydrodynamic characteristics. J Am Coll Cardiol 1984;3:313-20. [Crossref] [PubMed]
- Gray RJ, Chaux A, Matloff JM, et al. Bileaflet tilting disc and porcine aortic valve substitutes: In vivo hydrodynamic characteristics. J Am Coll Cardiol 1984;3:321-7. [Crossref] [PubMed]
- Walker PG, Yoganathan AP. In vitro pulsatile flow hemodynamics of five mechanical aortic heart valve prostheses. Eur J Cardiothorac Surg 1992;6 Suppl 1:S113-23. [Crossref] [PubMed]
- Dexter EU, Aluri S, Radcliffe RR, et al. In vivo demonstration of cavitation potential of a mechanical heart valve. ASAIO J 1999;45:436-41. [Crossref] [PubMed]
- Scotten LN, Siegel R. Thrombogenic potential of transcatheter aortic valve implantation with trivial paravalvular leakage. Ann Transl Med 2014;2:43. [PubMed]
- Scotten LN, Siegel R. Are anticoagulant independent mechanical valves within reach-fast prototype fabrication and in vitro testing of innovative bi-leaflet valve models. Ann Transl Med 2015;3:197. [PubMed]
- Vahidkhah K, Cordasco D, Abbasi M, et al. Flow-Induced Damage to Blood Cells in Aortic Valve Stenosis. Ann Biomed Eng 2016;44:2724-36. [Crossref] [PubMed]
- Jun BH, Saikrishnan N, Yoganathan AP. Micro particle image velocimetry measurements of steady diastolic leakage flow in the hinge of a St. Jude Medical® regent™ mechanical heart valve. Ann Biomed Eng 2014;42:526-40. [Crossref] [PubMed]
- von Knobelsdorff-Brenkenhoff F, Dieringer MA, Greiser A, et al. In vitro assessment of heart valve bioprostheses by cardiovascular magnetic resonance: four-dimensional mapping of flow patterns and orifice area planimetry. Eur J Cardiothorac Surg 2011;40:736-42. [PubMed]
- Yun BM, McElhinney DB, Arjunon S, et al. Computational simulations of flow dynamics and blood damage through a bileaflet mechanical heart valve scaled to pediatric size and flow. J Biomech 2014;47:3169-77. [Crossref] [PubMed]
- von Knobelsdorff-Brenkenhoff F, Trauzeddel RF, Barker AJ, et al. Blood flow characteristics in the ascending aorta after aortic valve replacement--a pilot study using 4D-flow MRI. Int J Cardiol 2014;170:426-33. [Crossref] [PubMed]
- Rademakers FE, Claus P. Imaging Hemodynamics: The Next Frontier for CMR. JACC: Cardiovascular Imaging 2014;7:927-9. [Crossref] [PubMed]
- Rowley JW, Finn AV, French PA, et al. Cardiovascular devices and platelet interactions: understanding the role of injury, flow, and cellular responses. Circ Cardiovasc Interv 2012;5:296-304. [Crossref] [PubMed]
- Xenos M, Girdhar G, Alemu Y, et al. Device Thrombogenicity Emulator (DTE)--design optimization methodology for cardiovascular devices: a study in two bileaflet MHV designs. J Biomech 2010;43:2400-9. [Crossref] [PubMed]


