Alleviation of gram-negative bacterial lung inflammation by targeting HECTD2
Lung injury remains a significant clinical problem worldwide. The nature and pathogenesis of the injury is highly multifactorial as it can be acute or chronic, triggered by bacteria, viruses, fungi, transfusions, sepsis, multiple fractures, aspiration and several other factors. In the case of invading pathogens and sepsis, the innate immune system may get overwhelmed, resulting in the secretion of large amounts of proinflammatory cytokines which mediate pulmonary edema, shock and potentially, multi-organ failure (1,2).
From this perspective, Coon and colleagues have unraveled a novel pathway in murine models of pneumonia induced by either the gram-negative bacterium Pseudomonas aeruginosa (PA) or the gram-negative endotoxin lipopolysaccharide (LPS) (3). This pathway centers around protein inhibitor of activated signal transducers and activators of transcription (STATs) (PIAS). The PIAS protein family members all play important roles in the regulation of various cellular events such as cell survival, cell migration and mediation of several signal transduction pathways (4). The cellular effects of PIAS1 are generally translated to down-regulation of distinct inflammatory pathways (4) and interestingly, PIAS1 knockout mice have been shown to display significantly elevated levels of proinflammatory cytokines (5). In addition to inhibiting STAT, PIAS1 also inhibits nuclear factor κB (NF-κB)-dependent gene activation (5-9). The paper by Coon et al. uncovered a previously unappreciated role for HECTD2, a ubiquitin E3 ligase, which is able to ubiquitinate and mediate the degradation of PIAS1 thereby rescuing STAT and NF-κB signaling and enabling pulmonary inflammation in mice. Furthermore, the authors performed a single-nucleotide polymorphism (SNP) database analysis and discovered a naturally occurring nonsynonymous G/C polymorphism (rs7081569) within HECTD2 (A19P) with an allele frequency of 8.5%. This HECTD2A19P was found to predominantly reside in the cytosol and had lost the ability to degrade PIAS1 protein in vitro as nuclear entry of HECTD2 was shown to be required for interaction with PIAS1. To further assess the contribution of HECTD2A19Pin vivo, mice were infected with a lentivirus encoding HECTD2WT (wildtype) or HECTD2A19P followed by challenging the mice with PA. In contrast to mice infected with HECTD2WT, mice infected with HECT2D2A19P failed to induce PA-mediated lung injury. As PA-induced pneumonia is also implicated in acute respiratory distress syndrome (ARDS) (10), the authors evaluated the HECT2D2A19P polymorphism in a cohort of 63 patients with or at-risk for ARDS. The results indicated that not a single patient carried the HECT2D2A19P polymorphism. They next investigated the inflammatory effects induced by HECTD2 in vivo and found that PIAS1 knockdown induced significant lung injury in mice as assessed by bronchial lavage protein concentrations, lavage cell counts, lavage cytokines and cell infiltrates. Subsequently, knockdown of HECTD2 ameliorated the PA-inflicted lung injury in vivo. Next, the authors cleverly searched for a small-molecule inhibitor of HECTD2 and as such identified compound BC-1382. They confirmed the binding as well as the inhibitory effect of BC-1382 towards HECTD2 and showed that BC-1382 also improved PIAS1 protein stability by increasing its half-life and by suppressing LPS-induced PIAS1 degradation. Importantly, BC-1382 also decreased the severity of the lung injury and cytokine levels in both murine LPS- and PA-induced pneumonia models as was assessed 18 hours after IP injection of the compound.
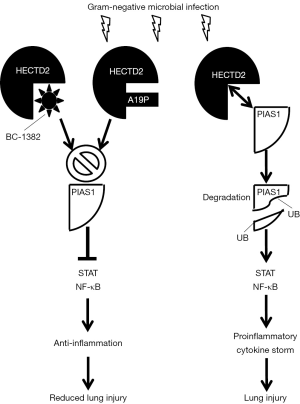
The findings by Coon et al. are summarized in Figure 1, which schematically shows that during microbial infection with gram-negative bacteria, HECTD2 targets PIAS1, resulting in PIAS1-ubiquitination (UB) and degradation. As a result, cytokine-driven inflammation is promoted resulting in lung injury. Compound BC-1382 can bind and inhibit HECTD2 and prevents PIAS1 degradation thereby shifting the balance towards PIAS1-induced anti-inflammatory signals which suppress secretion of proinflammatory cytokines and alleviates the lung injury. The mutated HECTD2A19P, however, mainly resides in the cytosol and is incapable of interacting with PIAS1 in the nucleus thus protecting individuals from lung injury induced by PIAS1-degradation.
The study by Coon et al. has greatly advanced the field and should be seen as a stepping stone towards deciphering the contribution of the HECTD2-PIAS1 pathway in other models of experimental lung injury. For instance, would this model also be relevant and therapeutically exploitable in a setting of gram-positive bacterial lung inflammation such as when inflicted by Streptococcus pneumonia which is in fact one of the main pathogens responsible for community-acquired pneumonia worldwide (11-14)? Similarly, the setting of viral or fungal-related pneumonia would also be interesting to assess the effects and the relevance of the HECTD2-PIAS interaction. Likewise, the contribution of the acute phase protein C-reactive protein (CRP) in this context would be interesting as it was recently shown that CRP can enhance transfusion-related acute lung injury (TRALI) in mice in part through the enhancement of the pro-inflammatory cytokine macrophage inflammatory protein (MIP)-2 (15), the murine ortholog of interleukin (IL)-8. Although the authors did assess HECTD2 polymorphisms in patients at-risk or suffering from ARDS, it would be insightful to perform these studies in other patient populations as well such as patients with or at-risk for (recurrent) pneumonia. Therapeutic compounds targeting HECTD2 may prove to be a valuable intervention especially in light of the increasing resistance to antibiotic regimens. As the authors noted, however, the safety profile and in vivo kinetics of such therapeutic compounds will need to be extensively characterized.
Overall, the paper by Coon et al. provides compelling data on the pathogenesis of gram-negative bacterial pneumonia with novel therapeutic insights. This may have the potential to eventually result in an attractive alternative for antibiotic-resistant pneumonia. These findings should stimulate further investigations into various disease models and patient cohorts of different types of lung injury.
Acknowledgements
This work was supported by grants from Health Canada and Canadian Blood Services (J.W.S.). R.K. is the recipient of a Post-Doctoral Fellowship from the Canadian Blood Services.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mallampalli RK, Coon TA, Glasser JR, et al. Targeting F box protein Fbxo3 to control cytokine-driven inflammation. J Immunol 2013;191:5247-55. [Crossref] [PubMed]
- Chen BB, Coon TA, Glasser JR, et al. A combinatorial F box protein directed pathway controls TRAF adaptor stability to regulate inflammation. Nat Immunol 2013;14:470-9. [Crossref] [PubMed]
- Coon TA, McKelvey AC, Lear T, et al. The proinflammatory role of HECTD2 in innate immunity and experimental lung injury. Sci Transl Med 2015;7:295ra109. [Crossref] [PubMed]
- Rytinki MM, Kaikkonen S, Pehkonen P, et al. PIAS proteins: pleiotropic interactors associated with SUMO. Cell Mol Life Sci 2009;66:3029-41. [Crossref] [PubMed]
- Liu B, Yang R, Wong KA, et al. Negative regulation of NF-kappaB signaling by PIAS1. Mol Cell Biol 2005;25:1113-23. [Crossref] [PubMed]
- Liu B, Shuai K. Targeting the PIAS1 SUMO ligase pathway to control inflammation. Trends Pharmacol Sci 2008;29:505-9. [Crossref] [PubMed]
- Liu B, Yang Y, Chernishof V, et al. Proinflammatory stimuli induce IKKalpha-mediated phosphorylation of PIAS1 to restrict inflammation and immunity. Cell 2007;129:903-14. [Crossref] [PubMed]
- Liu B, Liao J, Rao X, et al. Inhibition of Stat1-mediated gene activation by PIAS1. Proc Natl Acad Sci U S A 1998;95:10626-31. [Crossref] [PubMed]
- Liu Y, Zhang YD, Guo L, et al. Protein inhibitor of activated STAT 1 (PIAS1) is identified as the SUMO E3 ligase of CCAAT/enhancer-binding protein β (C/EBPβ) during adipogenesis. Mol Cell Biol 2013;33:4606-17. [Crossref] [PubMed]
- Meduri GU, Reddy RC, Stanley T, et al. Pneumonia in acute respiratory distress syndrome. A prospective evaluation of bilateral bronchoscopic sampling. Am J Respir Crit Care Med 1998;158:870-5. [Crossref] [PubMed]
- Prina E, Ranzani OT, Torres A. Community-acquired pneumonia. Lancet 2015;386:1097-108. [Crossref] [PubMed]
- Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax 2012;67:71-9. [Crossref] [PubMed]
- Drijkoningen JJ, Rohde GG. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect 2014;20 Suppl 5:45-51. [Crossref] [PubMed]
- Howard LS, Sillis M, Pasteur MC, et al. Microbiological profile of community-acquired pneumonia in adults over the last 20 years. J Infect 2005;50:107-13. [Crossref] [PubMed]
- Kapur R, Kim M, Shanmugabhavananthan S, et al. C-reactive protein enhances murine antibody-mediated transfusion-related acute lung injury. Blood 2015;126:2747-51. [Crossref] [PubMed]


