Is pulmonary artery pressure a trigger of adverse outcome in mitral regurgitation?
Mitral regurgitation (MR) is an important cause of morbidity and mortality in developed countries (1,2). The most common cause of MR is degenerative with an age-related epidemiological burden consisting of a peak incidence in patients over 70 years of age (1). Open surgical correction, using mitral valve repair or replacement, is currently accepted as the standard treatment of MR. Congestive heart failure symptoms or left ventricular (LV) dysfunction (EF <60% or end-systolic diameter >40 or >45 mm according to ACC/AHA and ESC guide-lines, respectively) are the suggested Class-I triggers for surgery (3,4). However, surgical treatment based on Class-I triggers may be characterized by a suboptimal post-operative outcome. Due to its long standing asymptomatic clinical course, the selection of optimal timing for surgery remains an important challenge (5-7). Due to the absence of randomized studies, current guidelines are based on the inference from observational studies or expert opinions. An ESC-position paper supports the organization of specialized valve clinics attempting optimal and individualized MR management (8). In the last few decades, the wide application of mitral valve repair has progressively changed the timing for surgery in patients with MR. Targeting valve lesion treatment, independent of symptoms and LV deterioration signs, mitral valve repair challenges the more conservative valve replacement approach, leading to early surgery in order to achieve an optimal post-operative outcome. The key points to consider are the predictability of repair using the best surgical strategy based on the functional mechanism of MR, the evaluation of MV repair efficacy before closing the chest, prediction and management of complications, and radicalization of MR treatment to achieve a durable repair without long-term recurrence or lesion progression. Mitral valve repair has been proposed for the treatment of MR without Class-I triggers when functional mitral anatomy matched with an experienced surgical team predicts a 95% rate of successful and durable repair with an expected surgical mortality <1%. Unlike the ACC/AHA statement, decision-making based on surgical reparability alone is not considered beneficial in the ESC recommendations (Class IIb). Although repair feasibility is considered a key point for early surgery in the real world the ultimate repair of degenerative MR is around 60%, with great inter-hospital variability related to team experience and intervention volume rate. The use of three-dimensional imaging may improve communication with surgeons, enabling them to predict a surgical strategy that might help close the gap between valve reparability and ultimate repair. New onset atrial fibrillation or resting pulmonary hypertension (PH) may be the facilitators of decision-making to perform mitral repair in asymptomatic MR with preserved LV function (Class IIa).
An important aspect of MR management is patient preference regarding the possibility to safely delay surgery in the absence of Class I-Trigger. Rest pulmonary artery hypertension is considered a harbinger of adverse outcome in asymptomatic MR and should be regarded as a non-deferrable condition for surgery (9-15). Resting systolic Pulmonary Artery Pressure (sPAP) >50 mmHg estimated by Doppler tricuspid regurgitant jet has been proposed as the threshold value triggering surgical treatment of asymptomatic MR patients. Mentias et al. in a recent retrospective observational study evaluated a large population with primary myxomatous MR and preserved LV function observed over a 3-year time frame that underwent mitral surgery based on class I or IIa indications (16). Although the incidence of adverse events is correlated with PH severity, this study also found that an sPAP value >35 mmHg (a lower value than the accepted threshold >50 mmHg) was significantly and independently associated with reduced long-term survival beyond the established triggers for surgery. Although it provides additional evidence on the prognostic role of PH, the proposed sPAP >35 mmHg for re-classification of MR at risk deserves some caveats, and should be confirmed in prospective cohort studies.
Several methodological issues should be addressed regarding the reliability of non-invasive sPAP measurement with peak Doppler tricuspid regurgitation velocity using the Bernoulli equation adding the estimated right atrial pressure. Unlike the use of a fixed value (10 mmHg) used in other studies, in this retrospective series right atrial pressure was estimated from the inferior vena cava (IVC) diameter and respiratory collapsibility as follows: 3 mmHg (IVC <2.1 cm, collapse with sniff >50%); 8 mmHg (IVC >2.1 cm, collapse >50%); and 15 mmHg (IVC >2.1 cm, collapse with sniff <50%). This approach could influence sPAP calculation and related threshold risk. Furthermore, the sample of an interpretable tricuspid regurgitant jet is crucial for sPAP assessment. Finally, right ventricular dysfunction may reduce the TR regurgitant jet leading to sPAP underestimation. From a pathophysiological point of view, the development of PH in MR patients should be regarded as a indirect sign of exhausted left atrium compliance leading to transmission of high pressure into the pulmonary circulatory bed (17,18). In addition to its mechanical properties, the natriuretic peptide release due to appendage stretching may modulate the intracavitary adaptation to mitral regurgitant volume and related sPAP (19). Consequently, we can depict a varying left atrial pressure/volume relationship arising from the balance between MR regurgitant volume, mechanical left atrial size and compliance, and neuropeptide release. A pathophysiological spectrum of MR may be extended from a low-compliant small left atrium, leading to early pulmonary hypertension and related symptoms, to very large high-compliant left atrium delaying pulmonary hypertension and symptom occurrence (Figure 1).
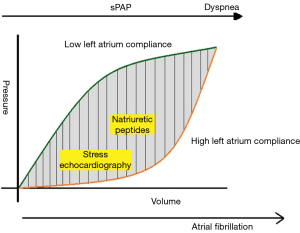
Exercise Echocardiography may induce a significant increase of sPAP in patient with normal or mild PH, providing an indirect sign of exhausted left atrial reserve (20,21). Natriuretic atrial peptides play an important role in modulating the impact of the regurgitant volume on left atrial pressure/volume relationship. Consequently, the evaluation of maladaptive exercise pulmonary pressure, together with natriuretic peptides, may add important information regarding the left atrial reserve to detect the decompensated phase and related risk in MR patients despite the absence of Class-I trigger. However, the highly compliant enlarged left atrium may be a misleading condition which can prevent the occurrence of MR-related PH and mask myocardial damage facilitating LV ejection. Consequently, the absence of elevated sPAP should not necessarily be considered a benign sign in the presence of a very enlarged left atrium. Finally, a careful clinical work-up should address the recognition of associated comorbidities that might provoke confounding PH independently of MR overload (22).
In conclusion, PH provides indirect evidence of exhaustion of left atrial reserve in MR patients and should be regarded as a trigger for surgical treatment of asymptomatic MR with preserved pump function indices. Further prospective studies are needed to establish the optimal rest-exercise cut-off to improve the optimal selection of timing for MR surgery.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11. [Crossref] [PubMed]
- Iung B, Vahanian A. Epidemiology of valvular heart disease in the adult. Nat Rev Cardiol 2011;8:162-72. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2438-88. [Crossref] [PubMed]
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC)1; European Association for Cardio-Thoracic Surgery (EACTS), Vahanian A, Alfieri O, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451-96.
- Goldstone AB, Patrick WL, Cohen JE, et al. Early surgical intervention or watchful waiting for the management of asymptomatic mitral regurgitation: a systematic review and meta-analysis. Ann Cardiothorac Surg 2015;4:220-9. [PubMed]
- Montant P, Chenot F, Robert A, et al. Long-term survival in asymptomatic patients with severe degenerative mitral regurgitation: a propensity score-based comparison between an early surgical strategy and a conservative treatment approach. J Thorac Cardiovasc Surg 2009;138:1339-48. [Crossref] [PubMed]
- Gillam LD, Marcoff L, Shames S. Timing of surgery in valvular heart disease: prophylactic surgery vs watchful waiting in the asymptomatic patient. Can J Cardiol 2014;30:1035-45. [Crossref] [PubMed]
- Lancellotti P, Rosenhek R, Pibarot P, et al. ESC Working Group on Valvular Heart Disease position paper--heart valve clinics: organization, structure, and experiences. Eur Heart J 2013;34:1597-606. [Crossref] [PubMed]
- Alexopoulos D, Lazzam C, Borrico S, et al. Isolated chronic mitral regurgitation with preserved systolic left ventricular function and severe pulmonary hypertension. J Am Coll Cardiol 1989;14:319-22. [Crossref] [PubMed]
- Magne J, Pibarot P, Sengupta PP, et al. Pulmonary hypertension in valvular disease: a comprehensive review on pathophysiology to therapy from the HAVEC Group. JACC Cardiovasc Imaging 2015;8:83-99. [Crossref] [PubMed]
- Le Tourneau T, Richardson M, Juthier F, et al. Echocardiography predictors and prognostic value of pulmonary artery systolic pressure in chronic organic mitral regurgitation. Heart 2010;96:1311-7. [Crossref] [PubMed]
- Barbieri A, Bursi F, Grigioni F, et al. Prognostic and therapeutic implications of pulmonary hypertension complicating degenerative mitral regurgitation due to flail leaflet: a multicenter long-term international study. Eur Heart J 2011;32:751-9. [Crossref] [PubMed]
- Ghoreishi M, Evans CF, DeFilippi CR, et al. Pulmonary hypertension adversely affects short- and long-term survival after mitral valve operation for mitral regurgitation: implications for timing of surgery. J Thorac Cardiovasc Surg 2011;142:1439-52. [Crossref] [PubMed]
- Yang H, Davidson WR Jr, Chambers CE, et al. Preoperative pulmonary hypertension is associated with postoperative left ventricular dysfunction in chronic organic mitral regurgitation: an echocardiographic and hemodynamic study. J Am Soc Echocardiogr 2006;19:1051-5. [Crossref] [PubMed]
- Le Tourneau T, Richardson M, Juthier F, et al. Echocardiography predictors and prognostic value of pulmonary artery systolic pressure in chronic organic mitral regurgitation. Heart 2010;96:1311-7. [Crossref] [PubMed]
- Mentias A, Patel K, Patel H, et al. Effect of Pulmonary Vascular Pressures on Long-Term Outcome in Patients With Primary Mitral Regurgitation. J Am Coll Cardiol 2016;67:2952-61. [Crossref] [PubMed]
- Le Tourneau T, Messika-Zeitoun D, Russo A, et al. Impact of left atrial volume on clinical outcome in organic mitral regurgitation. J Am Coll Cardiol 2010;56:570-8. [Crossref] [PubMed]
- Blondheim DS, Osipov A, Meisel SR, et al. Relation of left atrial size to function as determined by transesophageal echocardiography. Am J Cardiol 2005;96:457-63. [Crossref] [PubMed]
- Bergler-Klein J, Gyöngyösi M, Maurer G. The role of biomarkers in valvular heart disease: focus on natriuretic peptides. Can J Cardiol 2014;30:1027-34. [Crossref] [PubMed]
- Magne J, Lancellotti P, Piérard LA. Exercise pulmonary hypertension in asymptomatic degenerative mitral regurgitation. Circulation 2010;122:33-41. [Crossref] [PubMed]
- Lancellotti P, Fattouch K, La Canna G. Therapeutic decision-making for patients with fluctuating mitral regurgitation. Nat Rev Cardiol 2015;12:212-9. [Crossref] [PubMed]
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67-119. [Crossref] [PubMed]


