Remote cannulation and extracorporeal membrane oxygenation transport is safe in a newly established program
Background
The CESAR trial has demonstrated the potential role of extracorporeal membrane oxygenation (ECMO) in the treatment of severe acute respiratory distress syndrome (1,2). However, noteworthy in this trial was the observation that patients transported to ECMO centers, whether placed on extracorporeal support or not, had markedly improved survival when compared to those who remained at local referral centers. This study also demonstrated a remarkable risk to transport as 3 of 90 patients scheduled for transport expired prior to transport and 2 expired during transport.
In the most recent statistics (July 2016) from the Extracorporeal Life Support Organization (ELSO), ECMO was available at 310 centers reporting outcomes worldwide. Thus, while technological improvements have made the use of ECMO for cardiopulmonary support considerably easier than in prior years, the relatively small number of centers with the capability to support ECMO patients limits its utility for the vast majority of hospitals for whom routine use of ECMO is not feasible (3,4). Accordingly, it is arguably as important to safely facilitate the transfer of critically ill patients to regional ECMO centers as it is to cannulate them for extracorporeal support.
The medical literature includes multiple reports of the successful ECMO transport programs, mostly involving patients on veno-venous (VV) ECMO support (5-13). However, most of these reports originate in institutions with established internal ECMO programs. What remains unclear is the capacity of new ECMO programs to safely triage and transport patients meeting criteria for extracorporeal support from referral institutions. Our institution formally established an ECMO program on September 1, 2014 as described previously in (14). As our health system includes a number of hospitals spread across a large metropolitan area and provides quaternary referral care for a large segment of the southeastern United States, we realized that any formal ECMO program would require the ability to safely transport patients on or in need of extracorporeal support to a central unit specializing in such care. Accordingly, in conjunction with our perfusion department, a local ambulance service provider, and a separate fixed wing transport organization, we developed a transport component of our ECMO program in which all patients requiring transportation while on ECMO support would be accompanied by paramedics, a perfusionist, and a critical care physician. In the event patients would be transported using exceptionally high ventilator settings without ECMO support, a critical care physician would accompany the patient in addition to ambulance paramedics.
Methods
We retrospectively examined the charts of all patients transferred to Emory University Hospital (EUH) for VV or veno-arterial (VA) ECMO support from September 1, 2014 through November 30, 2015. Of note, while our institution had intermittently placed patients on ECMO support previously, the Emory ECMO Center was formally established on September 1, 2014. Included in our analysis were patients transferred by our service to EUH for consideration of ECMO support who did not ultimately require cannulation. Requests for ECMO support are routed through a system-wide transfer service. Contact is made first with the critical care attending in our cardiothoracic and vascular intensive care unit, which is where our program has chosen to place all patients requiring VA or VV ECMO support. As a result, the responsibilities for assessing ECMO suitability, cannulating remotely if needed, and safely transporting patients all lie with the critical care team which will manage the patient following transport. We believe this provides for improved continuity of care and resource allocation. All patients included in our analysis were transported by professional medical transportation agencies with whom our program contracts. All ECMO transports included at least one member of the critical care team (critical care medicine fellow or attending) and a member of the perfusion staff. In cases in which cannulation was deemed necessary for transport and in which cannulation was seen as potentially difficult or complicated, an attending surgeon from the cardiothoracic surgical division was also present. For transport of patients with potential need for VV ECMO support, our policy was not to transport patients with a P:F ratio less than 60 despite maximal therapy. For transport of patients with cardiac instability the decision to cannulate remotely vs. urgent transport without ECMO support was left to the referring institution.
All patients transported by our service had arterial and central venous access placed prior to our arrival. Intra-aortic balloon pumps (IABP) were placed at the discretion of the referring hospital; if in place, patients were transported to our facility with the IABP. Sedation was per the transporting team and typically consisted of benzodiazepines or propofol with neuromuscular blockade boluses as needed (including prior to cannulation and immediately prior to transport); analgesia was maintained with boluses or continuous infusions of opioids, most commonly fentanyl. Once on ECMO support, patients were placed on a lung protective ventilatory strategy, typically pressure control with PEEP at 10 cmH2O, drive pressure of 10 cmH2O above PEEP, respiratory rate of 10 with inspiratory time of 3 seconds, and minimum FiO2 required to maintain saturations above 90% (typically 40%). Inhaled pulmonary vasodilators (most commonly epoprostenol) were continued at the discretion of the managing intensivist.
Patients already on ECMO support were converted to support with the CARDIOHELP device (Maquet, Wayne, NJ, USA). For patients requiring remote cannulation by our team, support was initiated with the CARDIOHELP device (15-18). For VV ECMO support, cannulation was typically achieved with a RIJ 23 Fr return cannula (Medtronic BioMedicus, Minneapolis, MN, USA) and a 23–29 Fr femoral venous inflow cannula (Maquet, Wayne, NJ, USA). We chose to use a 2 cannula strategy for remote cannulation, as opposed to a dual lumen cannula, to avoid the need for fluoroscopy or transesophageal echocardiography. We did utilize portable surface ultrasonography for initial central venous access. During this time period, we did not offer remote cannulation for VA ECMO due to the small size of our team and potential for inadequate material and staff support required for arterial access.
ECMO support was continued until recovery or, in the case of patients supported on VA ECMO, implantation of permanent ventricular assist device (VAD). Patients successfully transitioned off ECMO to implanted VADs were considered alive for the purpose of this study only if they were also discharged from the hospital alive. ECMO support was discontinued and comfort care instituted when irreversible end organ damage (typically neurologic) was observed.
Results
From September 1, 2014 through November 30, 2015, a total of 28 patients were transported to our institution for consideration or management of ECMO. Of these, 24 were supported for at least some period of time on VA or VV ECMO; 10 out of these 24 patients survived to hospital discharge. Of the patients transported for ECMO consideration who were not cannulated, three out of four survived, including all three patients with severe acute lung injury.
Demographic information on all patients transported by our program is shown in Table 1. For patients receiving ECMO support, the mean duration of therapy at our institution was 153±129 hours. When compared to ECLS registry data from July 2015, patients transported on VV ECMO had a 45% survival to discharge (5 out of 11 patients) compared with 58% in the registry. When patients transferred for VV ECMO consideration, but who did not require cannulation, are included, survival to discharge was 57% (8 out of 14 patients). Patients transported on VA ECMO support by our service had a 38% (5 out of 13 patients) survival to discharge compared with 42% in the ECLS registry. Thus, survival rates comparable to international averages were observed in the first 15 months of our program in patients supported on both VA and VV ECMO.
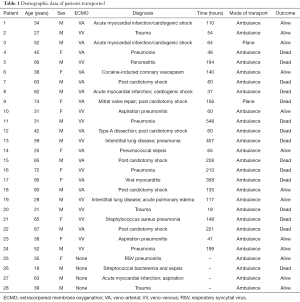
Full table
We encountered relatively few complications associated with transportation of patients. One patient was transported safely to our institution by our ECMO team but suffered a cardiac arrest on arrival to the ICU and could not be cannulated before he died (see patient 26 in Table 1). A second patient requiring VA ECMO at an outside facility was taken to the OR by surgeons at the facility and members of our team but could not be cannulated prior to evidence of massive neurologic injury and thus was not transported back. A third patient was deemed a candidate for VA ECMO support but surgical support was unavailable when our team arrived; cannulation did not take place and the patient remained at the referring institution. A total of four mechanical complications occurred during the 15 months period as well. These included a flat tire (which necessitated the transfer of the patient from one ambulance to another on the side of the interstate highway); defective ambulance batteries which delayed transport until a second ambulance was dispatched; partially filled ambulance oxygen tanks; and transport ventilator malfunction. However, there were no adverse events associated with any of these complications. In summary, the establishment of ECMO transport capability with a brand new program was not associated with any transportation-related morbidity or mortality.
Discussion
Our data suggest that newly established ECMO programs can begin the transport of critically ill patients on or in need of extracorporeal support from referring hospitals with an acceptable rate of complications and outcomes equivalent to established national and international ECMO centers. In reviewing our own data, overall outcomes for patients on placed on VV and VA ECMO at our center were somewhat lower than that observed in other programs. This was not seen with our transport populations. Two local patient populations drove this increased mortality: patients with severe immunosuppression due to bone marrow or stem cell transplantation and patients with post cardiotomy/post cardiopulmonary bypass shock. Patients being referred for respiratory support at outside facilities typically were not immunocompromised. Additionally, patients transferred to us for VA ECMO support more frequently had either (I) pathology likely to improve with supportive care or (II) the opportunity to transition to implantable mechanical support not available at the referring institutions.
One limitation of our study is the lack of a comparison or control group. That is, we have no way of assessing what the outcomes of our patients would have been had they remained at the local referral centers. This reflects the difficulty in testing outcomes from extracorporeal support in a randomized, controlled manner. We also cannot comment on the success of ECMO management at our center vs. outcomes had the patients been managed on ECMO at the referring centers. However, the fact that all transfers were made at the request of the transferring facility suggests that local capacity to care for these patients had been exceeded.
In conclusion, the data collected in this study demonstrates that transport of patients in a well-organized new program is safe and effective. Patients are able to be transported by a multidisciplinary team without high risk of morbidity or in transit mortality. Further research is required to determine if such transport improves overall survival to discharge.
Acknowledgements
The authors wish to thank the staff and physicians of the cardiothoracic intensive care unit at Emory University Hospital for their tireless care of these patients both during transport and after arrival in our ICU. We acknowledge the assistance of Duc Nguyen, MD whose surgical expertise was essential for the cannulation of many of the patients in our study. Lastly, we would like to thank the staff of the Emory University Perfusion and Respiratory Therapy Departments who were part of every transport and who were instrumental in maintaining and supporting our program in house.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63. [Crossref] [PubMed]
- Combes A, Bacchetta M, Brodie D, et al. Extracorporeal membrane oxygenation for respiratory failure in adults. Curr Opin Crit Care 2012;18:99-104. [Crossref] [PubMed]
- Wallace DJ, Angus DC, Seymour CW, et al. Geographic access to high capability severe acute respiratory failure centers in the United States. PLoS One 2014;9:e94057. [Crossref] [PubMed]
- Combes A, Brodie D, Bartlett R, et al. Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med 2014;190:488-96. [Crossref] [PubMed]
- Biscotti M, Agerstrand C, Abrams D, et al. One Hundred Transports on Extracorporeal Support to an Extracorporeal Membrane Oxygenation Center. Ann Thorac Surg 2015;100:34-9; discussion 39-40. [Crossref] [PubMed]
- Bryner B, Cooley E, Copenhaver W, et al. Two decades' experience with interfacility transport on extracorporeal membrane oxygenation. Ann Thorac Surg 2014;98:1363-70. [Crossref] [PubMed]
- Ciapetti M, Cianchi G, Zagli G, et al. Feasibility of inter-hospital transportation using extra-corporeal membrane oxygenation (ECMO) support of patients affected by severe swine-flu(H1N1)-related ARDS. Scand J Trauma Resusc Emerg Med 2011;19:32. [Crossref] [PubMed]
- Forrest P, Ratchford J, Burns B, et al. Retrieval of critically ill adults using extracorporeal membrane oxygenation: an Australian experience. Intensive Care Med 2011;37:824-30. [Crossref] [PubMed]
- Isgrò S, Patroniti N, Bombino M, et al. Extracorporeal membrane oxygenation for interhospital transfer of severe acute respiratory distress syndrome patients: 5-year experience. Int J Artif Organs 2011;34:1052-60. [Crossref] [PubMed]
- Javidfar J, Brodie D, Takayama H, et al. Safe transport of critically ill adult patients on extracorporeal membrane oxygenation support to a regional extracorporeal membrane oxygenation center. ASAIO J 2011;57:421-5. [Crossref] [PubMed]
- Michaels AJ, Hill JG, Long WB, et al. Adult refractory hypoxemic acute respiratory distress syndrome treated with extracorporeal membrane oxygenation: the role of a regional referral center. Am J Surg 2013;205:492-8; discussion 498-9. [Crossref] [PubMed]
- Roch A, Hraiech S, Masson E, et al. Outcome of acute respiratory distress syndrome patients treated with extracorporeal membrane oxygenation and brought to a referral center. Intensive Care Med 2014;40:74-83. [Crossref] [PubMed]
- Wagner K, Sangolt GK, Risnes I, et al. Transportation of critically ill patients on extracorporeal membrane oxygenation. Perfusion 2008;23:101-6. [Crossref] [PubMed]
- Moll V, Teo EY, Grenda DS, et al. Rapid Development and Implementation of an ECMO Program. ASAIO J 2016;62:354-8. [Crossref] [PubMed]
- Haneya A, Philipp A, Foltan M, et al. First experience with the new portable extracorporeal membrane oxygenation system Cardiohelp for severe respiratory failure in adults. Perfusion 2012;27:150-5. [Crossref] [PubMed]
- Alwardt CM, Wilson DS, Alore ML, et al. Performance and Safety of an Integrated Portable Extracorporeal Life Support System for Adults. J Extra Corpor Technol 2015;47:38-43. [PubMed]
- Philipp A, Arlt M, Amann M, et al. First experience with the ultra compact mobile extracorporeal membrane oxygenation system Cardiohelp in interhospital transport. Interact Cardiovasc Thorac Surg 2011;12:978-81. [Crossref] [PubMed]
- Raspé C, Rückert F, Metz D, et al. Inter-hospital transfer of ECMO-assisted patients with a portable miniaturized ECMO device: 4 years of experience. Perfusion 2015;30:52-9. [Crossref] [PubMed]


