The association between bacteria and urinary stones
Urinary stone disease (USD) is an expanding clinical problem
USD is an expanding problem. Approximately 10% of people will have a urinary stone during their lifetime (1). The United States health care burden from USD is immense with 185,000 hospitalizations, 2 million outpatient visits and 2.1 billion dollars expended annually for management (2-4). Historically, a key component in urinary stone formation is supersaturation, a process by which the concentration of substances in urine, such as calcium and oxalate, exceed the limits of their solubility (5). However, considerable overlap in urine chemistries exists between individuals with and without USD (6-8). Furthermore, supersaturation with calcium oxalate (CaOx) or calcium phosphate (CaPhos) is not different in recurrent USD patients compared to controls (9). Thus, although supersaturated urine is a risk factor, alone it is insufficient for stone formation. This conclusion is supported by the knowledge that treatment with dietary modifications, increased fluid intake, citrate salts and/or thiazide diuretics to reduce urine CaOx supersaturation only moderately improves recurrence rates (10-12). Despite these treatment strategies, USD prevalence in US adults and children has recently increased by 40% and 23%, respectively (13,14). Identification of other factors that contribute to CaOx and/or CaPhos stone formation (lithogenesis) is a critical need. The bacterial contribution to USD formation has long been recognized. Magnesium-ammonium-phosphate (struvite) stones (a conglomeration of bacteria, crystals and protein matrix) form due to urinary tract infection (UTI) with urease-producing bacteria (15). However, struvite accounts for only 4% of urinary stones. In contrast, CaOx is found in greater than 60% of urinary stones, while CaPhos is found in more than 40% (16). The objective of this review is to discuss emerging evidence indicating that bacteria might contribute to CaOx and/or CaPhos USD.
USD relevance of the microbiota
A number of conditions, including obesity, childhood onset asthma, and cardiovascular disease are associated with disruptions to the microbiota—the microbes that inhabit the human body (17,18). In USD, most published microbiota research papers have focused on the intestinal flora. For example, intestinal colonization with Oxalobacter formigenes, which degrades oxalate, is associated with lower urine oxalate levels and fewer recurrent stones; though a protective effect of exogenous Oxalobacter remains unconfirmed (19). Emerging evidence indicates that bacteria are present in and contribute to vascular calcifications (20,21). Therefore, it is possible that bacteria also are present in and contribute to urinary calcifications.
UTI and USD occur in the same patients
In a whole population survey of Taiwan, a UTI history was the most common associated condition in children with newly diagnosed USD (22). Overall, 34% of children (44% of females and 24% of males) diagnosed with USD had a history of UTIs (22). In contrast, the reported childhood UTI prevalence is only 8% (23). The association between UTI and USD extends into adulthood. For example, in a retrospective review of 1,325 adults admitted for USD to a Swedish hospital over a 7-year period, 28% had a positive standard urine culture (24).
Bacteria can be cultured from urinary stones
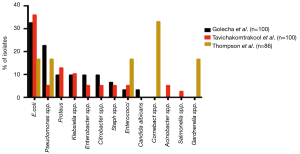
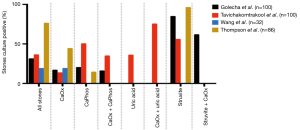
In addition to the aforementioned association between culture-positive urine and USD, bacteria can be cultured from the stones themselves. Studies from 1973 to the present in both Asia and North America have demonstrated that bacteria can be isolated from approximately 15–70% of stones following clinical culture (Figure 1) (25-28). When limited to CaOx stones, 13% to 44% of cultures were positive. E. coli and Pseudomonas spp. were the most common bacteria isolated from stone cultures, followed by the urease-splitting bacteria typically involved in struvite stone formation (Figure 2) (26-28). However, the reported results may under-represent the bacteria in urinary stones because the standard urine culture protocols used by clinical microbiologists are designed to identify clinical infections by known uropathogens. These protocols are not conducive to growth by slow growing, fastidious or anaerobic bacteria, which comprise the vast majority of bacteria found in urine (29).
Sequencing and new culture techniques expand our knowledge of the urinary tract microbiota
To expand detection and identification of bacteria in low biomass samples such as urine, we developed enhanced quantitative urine culture (EQUC) (30). EQUC incorporates more urine volume and a longer incubation period, along with multiple media types and atmospheric conditions (30). In addition to enhanced culture techniques such as EQUC, 16S rRNA gene sequencing of bacterial DNA has resulted in a paradigm shift in our ability to study the microbiome (the genomes of the microbiota). 16S rRNA gene sequencing and enhanced culture are complementary, as the sequencing identifies bacteria that cannot be cultured but generally cannot classify below the genus level, while enhanced culture actually isolates the bacteria. These isolates can then be identified more precisely (down to the species and even strain level) and, most importantly, can be used to study biological relevance.
Bacteria can be sequenced from urinary stones
In 2015, we published a microbial study of five kidney stones along with both upper tract urine and bladder urine obtained during the stone removal procedures (31). The patients’ ages were between 12 and 29 years; four of them had documented CaOx stones (31).
In each stone, 16S rRNA gene sequencing identified multiple bacteria. Some of these bacteria were expected and consistent with historical stone cultures (29) and/or past sequencing reports of human urine (32-36). Expected bacteria included Pseudomonas, Gardnerella, Lactobacillus and Enterobactericeae, a large family that includes E. coli (31). Other bacteria were unexpectedly detected, particularly Brucella (31). Despite our best efforts, we were unable to successfully complete targeted sequencing of this organism and thus cannot confirm its identity. However, we speculate that it was actually Ochrobactrum, a close relative of Brucella that is often indistinguishable from Brucella by 16S sequencing (37). Intriguingly, Ochrobactrum has been identified in the urine (38).
EQUC isolated bacteria from only two stones. In each case, however, the isolated organism was consistent with the most dominant organism identified by sequencing. In one stone, Pseudomonas was the dominant organism by both EQUC and sequencing. In the second stone, EQUC isolated E. coli, while sequencing identified the dominant organism as a member of the family Enterobactericeae, which includes E. coli (31). Since the EQUC-positive stones were the largest (>15 mm), it is possible that stones of a certain size are needed to obtain enough biomass for a positive EQUC result (31).
From each individual, the bacteria detected by sequencing were often similar in the stone and urine samples, but the relative proportions differed. For example, in one individual, both Enterbactericeae and Gardnerella were identified in the stone and bladder urine. However, this was not always true, as Enterobactericeae dominated another individual’s stone while Gardnerella dominated their urine (31).
Although this initial study did demonstrate that 16S rRNA gene sequencing can consistently detect bacterial DNA in urinary stones, studies with larger numbers of patients are needed to comprehensively characterize the bacteria typically present in urinary stones.
Pyelonephritis and CaOx nephropathy potentiate each other
Whereas 16S rRNA gene sequencing and EQUC consistently detected the presence of bacteria in the limited number of urinary stones that we analyzed, it is not known whether the association between bacteria and USD is causal, disease modifying or merely coincidental. To further evaluate the association between USD and bacteria, we compared the renal bacterial burden and CaOx deposit number per cross section in mice with CaOx nephropathy and pyelonephritis by transurethral inoculation of uropathogenic E. coli (UPEC) induced alone and in combination (31). The combination of CaOx and experimental pylenonephritis resulted in a 130-fold higher bacterial burden than experimental alone (31). Conversely, the CaOx deposit number normalized to mean cross section area was 2.7-fold higher when both CaOx and UPEC were present compared to CaOx nephropathy alone (31).
Mechanisms by which bacteria may contribute to USD
If bacteria do indeed contribute to CaOx USD, what are the potential mechanisms? One possibility is that bacteria adhere to crystals. This mechanism is supported by findings that some bacteria selectively aggregate to certain crystal types but not others and that bacteria are associated with an increased number of crystal-crystal agglomerations. Compared to CaOx dihydrate and control silicon dioxide crystals, increased bacterial aggregation was noted around CaOx monohydrate crystals (31). Others have reported similar results. For example, Chutipongtanate and colleagues demonstrated that bacteria such as E. coli and Klebsiella pneumonia increased the number of CaOx crystal aggregates compared to blank or intact red blood cell control (39). Another way that bacteria could contribute to USD is bacterial production of citrate lyase, which could decrease the urine citrate levels that lead to supersaturated urine and crystal formation. In support of this potential mechanism is the finding of De Ferrari and colleagues, who demonstrated mean urine citrate, was 2-fold lower in 17 standard urine culture-positive patients compared to 30 standard urine culture-negative patients (40). Lastly, bacteria-crystal aggregates may bind to the tubular epithelium resulting in expression of stone matrix proteins in either renal tubular epithelium or inflammatory cells. The stone matrix proteins may be the component that, at least in part, differentiates crystalluria from progression to stone formation. In our murine CaOx nephropathy model, the combination of CaOx and UPEC significantly increased S100A9 mRNA expression over control, CaOx alone or UPEC alone by a factor >50-fold. A similar pattern was seen with osteopontin.
Conclusions
Bacteria and USD are clinically associated because they often occur in the same patients and USD patients often have positive urine and/or stone cultures. Whether they are mechanistically associated is an emerging research topic. Selective aggregation to some crystal types seen in USD patients, increased crystal clumping in the presence of bacteria, bacteria-induced lower urine citrate levels and increased CaOx deposits and stone matrix protein expression when bacteria are present as opposed to CaOx deposits alone are initial findings that might provide the basis to begin to unravel the reasons for the clinical USD-bacteria associations. Important future directions will include: (I) sequencing a larger number of stones to determine if the bacteria present are consistent between stone composition and other covariates such as sex and age and (II) devising model systems to test mechanistic overlap between USD and the bacteria isolated from urinary stones.
Acknowledgements
John Ketz’s review of the manuscript is acknowledged. AL Schwaderer has consulted for Allena Pharmaceuticals. AJ Wolfe is the Principal Investigator of Investigator Initiated Studies funded by Astellas Scientific and Medical Affairs and by Kimberly Clark Corporation.
Funding: AJ Wolfe is supported by NIH grants P20 DK108268 and R01 DK104718. AL Schwaderer is funded by NIH grants R01 DK106286 and R01 AG050801. Our funding sources have had no role in design or conduct of our studies; collection, management, analysis, and interpretation of our data; or in preparation, review, or approval of this or any other manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pak CY. Kidney stones. Lancet 1998;351:1797-801. [Crossref] [PubMed]
- Kozak LJ, Hall MJ, Owings MF. National Hospital Discharge Survey: 2000 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13 2002.1-194. [PubMed]
- DeFrances CJ, Hall MJ. 2005 National Hospital Discharge Survey. Adv Data 2007.1-19. [PubMed]
- Dickson KA, Haigis MC, Raines RT. Ribonuclease inhibitor: structure and function. Prog Nucleic Acid Res Mol Biol 2005;80:349-74. [Crossref] [PubMed]
- Coe FL, Parks JH, Asplin JR. The pathogenesis and treatment of kidney stones. N Engl J Med 1992;327:1141-52. [Crossref] [PubMed]
- Lande MB, Varade W, Erkan E, et al. Role of urinary supersaturation in the evaluation of children with urolithiasis. Pediatr Nephrol 2005;20:491-4. [Crossref] [PubMed]
- Lemann J Jr, Pleuss JA, Worcester EM, et al. Urinary oxalate excretion increases with body size and decreases with increasing dietary calcium intake among healthy adults. Kidney Int 1996;49:200-8. [Crossref] [PubMed]
- Curhan GC, Willett WC, Speizer FE, et al. Twenty-four-hour urine chemistries and the risk of kidney stones among women and men. Kidney Int 2001;59:2290-8. [Crossref] [PubMed]
- Borghi L, Guerra A, Meschi T, et al. Relationship between supersaturation and calcium oxalate crystallization in normals and idiopathic calcium oxalate stone formers. Kidney Int 1999;55:1041-50. [Crossref] [PubMed]
- Borghi L, Meschi T, Amato F, et al. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: a 5-year randomized prospective study. J Urol 1996;155:839-43. [Crossref] [PubMed]
- Tekin A, Tekgul S, Atsu N, et al. Oral potassium citrate treatment for idiopathic hypocitruria in children with calcium urolithiasis. J Urol 2002;168:2572-4. [Crossref] [PubMed]
- Fernández-Rodríguez A, Arrabal-Martín M, García-Ruiz MJ, et al. The role of thiazides in the prophylaxis of recurrent calcium lithiasis. Actas Urol Esp 2006;30:305-9. [PubMed]
- Stamatelou KK, Francis ME, Jones CA, et al. Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney Int 2003;63:1817-23. [Crossref] [PubMed]
- Sas DJ, Hulsey TC, Shatat IF, et al. Increasing incidence of kidney stones in children evaluated in the emergency department. J Pediatr 2010;157:132-7. [Crossref] [PubMed]
- Flannigan R, Choy WH, Chew B, et al. Renal struvite stones--pathogenesis, microbiology, and management strategies. Nat Rev Urol 2014;11:333-41. [Crossref] [PubMed]
- Pak CY, Poindexter JR, Adams-Huet B, et al. Predictive value of kidney stone composition in the detection of metabolic abnormalities. Am J Med 2003;115:26-32. [Crossref] [PubMed]
- Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57-63. [Crossref] [PubMed]
- Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027-31. [Crossref] [PubMed]
- Kaufman DW, Kelly JP, Curhan GC, et al. Oxalobacter formigenes may reduce the risk of calcium oxalate kidney stones. J Am Soc Nephrol 2008;19:1197-203. [Crossref] [PubMed]
- Clifford A, Hoffman GS. Evidence for a vascular microbiome and its role in vessel health and disease. Curr Opin Rheumatol 2015;27:397-405. [Crossref] [PubMed]
- Curran SA, Hollan I, Erridge C, et al. Bacteria in the adventitia of cardiovascular disease patients with and without rheumatoid arthritis. PLoS One 2014;9:e98627. [Crossref] [PubMed]
- Huang WY, Chen YF, Chen SC, et al. Pediatric urolithiasis in Taiwan: a nationwide study, 1997-2006. Urology 2012;79:1355-9. [Crossref] [PubMed]
- Shaikh N, Morone NE, Bost JE, et al. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J 2008;27:302-8. [Crossref] [PubMed]
- Holmgren K, Danielson BG, Fellström B, et al. The relation between urinary tract infections and stone composition in renal stone formers. Scand J Urol Nephrol 1989;23:131-6. [Crossref] [PubMed]
- Wang X, Krambeck AE, Williams JC Jr, et al. Distinguishing characteristics of idiopathic calcium oxalate kidney stone formers with low amounts of Randall's plaque. Clin J Am Soc Nephrol 2014;9:1757-63. [Crossref] [PubMed]
- Tavichakorntrakool R, Prasongwattana V, Sungkeeree S, et al. Extensive characterizations of bacteria isolated from catheterized urine and stone matrices in patients with nephrolithiasis. Nephrol Dial Transplant 2012;27:4125-30. [Crossref] [PubMed]
- Thompson RB, Stamey TA. Bacteriology of infected stones. Urology 1973;2:627-33. [Crossref] [PubMed]
- Golechha S, Solanki A. Bacteriology and chemical composition of renal calculi accompanying urinary tract infection. Indian J Urol 2001;17:111-7.
- Price TK, Dune T, Hilt EE, et al. The Clinical Urine Culture: Enhanced Techniques Improve Detection of Clinically Relevant Microorganisms. J Clin Microbiol 2016;54:1216-22. [Crossref] [PubMed]
- Hilt EE, McKinley K, Pearce MM, et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol 2014;52:871-6. [Crossref] [PubMed]
- Barr-Beare E, Saxena V, Hilt EE, et al. The Interaction between Enterobacteriaceae and Calcium Oxalate Deposits. PLoS One 2015;10:e0139575. [Crossref] [PubMed]
- Pearce MM, Hilt EE, Rosenfeld AB, et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. MBio 2014;5:e01283-14. [Crossref] [PubMed]
- Thomas-White K, Fok CS, Mueller ER, et al. Pre-Operative Urinary Microbiome Reveals Post-Operative Urinary Tract Infection Risk. Neurourology and Urodynamics 2015;34:S21-S2.
- Wolfe AJ, Toh E, Shibata N, et al. Evidence of uncultivated bacteria in the adult female bladder. J Clin Microbiol 2012;50:1376-83. [Crossref] [PubMed]
- Nelson DE, Van Der Pol B, Dong Q, et al. Characteristic male urine microbiomes associate with asymptomatic sexually transmitted infection. PLoS One 2010;5:e14116. [Crossref] [PubMed]
- Fouts DE, Pieper R, Szpakowski S, et al. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J Transl Med 2012;10:174. [Crossref] [PubMed]
- Velasco J, Romero C, López-Goñi I, et al. Evaluation of the relatedness of Brucella spp. and Ochrobactrum anthropi and description of Ochrobactrum intermedium sp. nov., a new species with a closer relationship to Brucella spp. Int J Syst Bacteriol 1998;48:759-68. [Crossref] [PubMed]
- Qasimyar H, Hoffman MA, Simonsen KA. Late-onset Ochrobactrum anthropi sepsis in a preterm neonate with congenital urinary tract abnormalities. J Perinatol 2014;34:489-91. [Crossref] [PubMed]
- Chutipongtanate S, Sutthimethakorn S, Chiangjong W, et al. Bacteria can promote calcium oxalate crystal growth and aggregation. J Biol Inorg Chem 2013;18:299-308. [Crossref] [PubMed]
- De Ferrari ME, Macaluso M, Brunati C, et al. Hypocitraturia and Ureaplasma urealyticum urinary tract infection in patients with idiopathic calcium nephrolithiasis. Nephrol Dial Transplant 1996;11:1185. [Crossref] [PubMed]