Optimum treatment for chronic obstructive pulmonary disease exacerbation prevention
An exacerbation of chronic obstructive pulmonary disease (COPD) is defined as an acute worsening of symptoms greater than the usual day to day variation that requires increased pharmacotherapy (1). Some COPD patients suffer with exacerbations regularly, and the history of previous exacerbations is the best predictor for the risk of future exacerbations (2). The Global initiative for chronic Obstructive Lung Disease (GOLD) uses a threshold of ≥ two exacerbations or one hospitalization in the last year to identify patients with an increased risk of future exacerbations (3).
The initial inhaled pharmacological treatment options for exacerbation prevention recommended by GOLD include monotherapy with a long acting muscarinic antagonist (LAMA) or combination therapy with an inhaled corticosteroid and long acting beta 2 agonist (ICS/LABA). Both of these treatments reduce exacerbation rates, and the investigating new standards for prophylaxis in reducing exacerbations (INSPIRE) clinical trial conducted in severe COPD patients with forced expiratory volume 1 (FEV1) <50% predicted and an exacerbation history demonstrated that these treatments had similar effects on exacerbation prevention (4). In real life, ICS/LABA combinations are widely used to treat COPD, as the combination of a long acting bronchodilator with an anti-inflammatory drug provides a convenient option to address different components of COPD including exacerbation prevention.
A recent advance in COPD treatment is the development of combination inhalers containing a LABA and LAMA. These dual bronchodilator combinations have greater effects on lung function compared to long acting bronchodilator monotherapies (5). The benefits of dual bronchodilator combinations on patient reported outcomes (PROs) have been debated, as some studies have shown small effects compared to monotherapies when considering group mean data (6-8). However, many of these studies were focused on lung function as a primary endpoint, and were not specifically designed or statistically powered to evaluate PROs. In contrast, clinical trials with PROs as the primary endpoint (9,10), pooled analysis (11)that increase statistical power and responder analysis (5) have demonstrated clinical benefits for dual bronchodilators compared to monotherapies. The early studies with LAMA/LABA combinations did not specifically investigate the effects in COPD patients with a history of exacerbations. This gap in knowledge was filled by the SPARK study, which recruited severe COPD patients with an exacerbation history, and demonstrated superiority of LAMA/LABA over LAMA monotherapy for exacerbation reduction (12). The growing evidence for the efficacy of LAMA/LABA combinations has encouraged many national guidelines to advocate the use of these drugs (13).
There are now two different types of combination inhaler available for COPD treatment (LABA/LAMA and ICS/LABA). A number of studies have compared these combinations in populations not enriched for frequent exacerbators. In these “low risk” populations, LAMA/LABA combinations have consistently demonstrated greater improvements in lung function than ICS/LABA, and there is also evidence of a symptomatic benefit (summarized in Table 1). The LANTERN study (17), conducted in COPD patients with no exacerbations or one exacerbation in the previous year, showed that indacaterol-glycopyrronium (Ind-Gly) caused a 31% risk reduction in the rate of moderate to severe exacerbations compared to salmeterol-fluticasone (SFC) over 26 weeks. The majority of patients in these studies were at low risk of future exacerbations, and an important question is how LABA/LAMA and ICS/LABA combinations compare in patients at high risk of exacerbations.
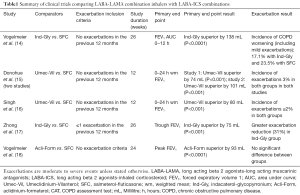
Full table
The FLAME (19) study was designed to compare the effects of Ind-Gly and SFC on COPD exacerbations. This was a double blind, double dummy, parallel group; 52 weeks randomized controlled trial (RCT) involving 3,362 randomized subjects in 43 countries. The primary aim was to demonstrate the non-inferiority of Ind-Gly to SFC with respect to the rate of exacerbations. Subjects with an FEV1 25–60% predicted and a history of at least 1 exacerbation in the preceding year were included. Subjects were treated with tiotropium during a 4-week run in period before randomisation.
An electronic dairy was used to record symptoms twice daily, in order to diagnose exacerbations using similar symptom criteria to those proposed by Anthonisen et al. (20). Patients were prompted to contact the trial site by alerts triggered by the electronic dairy when the criteria were met. Exacerbation severity was categorized as moderate when treatment with antibiotics and/or oral corticosteroids was required or severe when hospital admission or attendance at the emergency room for longer than 24 hours occurred. Exacerbations were classified as mild when symptom worsening did not require antibiotics and/or oral corticosteroids. The annual rate of all exacerbations in the Ind-Gly and SFC groups were 3.59 and 4.03 respectively, with an 11% lower rate of exacerbations with Ind-Gly treatment. This rate ratio met the primary endpoint non-inferiority analysis, allowing further analysis demonstrating that Ind-Gly was superior to SFC (P=0.003). Ind-Gly was superior to SFC for a range of secondary endpoints such as time to first exacerbation (71 vs. 51 days, P<0.001), annual rate of moderate to severe COPD exacerbations (0.98 vs. 1.19, 17% reduction P<0.001), time to first moderate to severe exacerbation (127 vs. 87 days, 22% lower risk, P<0.001) change in pre dose trough FEV1 from baseline (62 mL improvement P<0.001), and improvements in quality of life. The risk of pneumonia was significantly lower with Ind-Gly compared to SFC (3.2% vs. 4.8% respectively, P=0.02).
This FLAME study results show a consistent pattern of clinical benefit on exacerbations, lung function and health status in favour of dual bronchodilator over ICS/LABA treatment. Is this sufficient evidence to firmly recommend LABA/LAMA treatments over ICS/LABA in COPD patients with an exacerbation history? Probably not, as it would be preferable to see this result replicated, ideally using other drugs of the same classes in order to understand if this is a class effect. Also, it is important to note that the FLAME study did not use the GOLD definition of the number of moderate exacerbations (≥2) to be classified as high risk for inclusion in the study. The majority of COPD subjects (approximately 80%) in this study had one exacerbation in the preceding year. A subgroup analysis of patients with history of ≥2 exacerbations in the previous year yielded a rate ratio of 0.85 favouring Ind-Gly, although the confidence interval crossed one indicating no difference between treatments. The smaller sample size for this subgroup means that caution is required when interpreting this sub analysis, and ideally a study with a larger sample size could address this important patient group. However, there are practical difficulties in recruiting such COPD patients into clinical trials, as they often have more severe disease and are less willing to take part in studies that involve a step down in treatment, for example during the run-in period. Similarly, COPD patients with FEV1 <25% predicted were excluded from the FLAME study, so it is not possible to draw any conclusions about the comparison of these combination inhalers in very severe COPD. It is important that practicing clinicians who read clinical trials should be aware of the characteristics of the study population, as caution often needs to be applied when trying to extrapolate the findings beyond the population enrolled.
The recording of mild exacerbations in the FLAME study differs from many clinical trials that have focused only on moderate to severe events. The importance of mild exacerbations is unclear, as some of these events are likely to be genuinely minor worsening with rapid recovery, while other events may be more severe and prolonged, being more similar to moderate exacerbations but without receiving antibiotics and/or oral corticosteroids.
The FLAME study authors state that the rate of exacerbations was not significantly different between subjects with baseline peripheral blood eosinophil counts ≥2% and <2%. There is growing evidence that higher peripheral blood eosinophil counts predict an increased effect of ICS on exacerbations, mainly from studies comparing ICS/LABA to LABA (21,22). The different results in FLAME could be partly due to the fact that FLAME compares ICS to LAMA, with both groups receiving LABA, in contrast to previous eosinophil analysis which compares ICS to placebo (with both groups receiving LABA). Furthermore, it would be interesting to see the FLAME eosinophil analysis using higher blood eosinophil cut-off values where one would expect the ICS effect to increase.
Clinical trials generally report group mean data. However, real world clinical practice deals with individual treatment decisions. It is clear from FLAME that many COPD patients in both treatment arms continued to experience exacerbations during the study. What would be the next step for these individuals? An option would be step up to triple therapy with ICS plus LABA plus LAMA. A recent COPD study has clearly shown the benefits of triple therapy delivered in a single inhaler versus ICS/LABA on exacerbation rates (22). While the FLAME study provides evidence that LABA-LAMA combinations can be used successfully to prevent exacerbations, it also underscores that combination inhalers with two active medicines are insufficient for many patients.
There is an increasing focus on personalized medicine in COPD, tailoring therapies towards the individual characteristics (23). Until a few years ago, we had one combination inhaler (ICS/LABA) to treat COPD. Now we also have LABA/LAMA and ICS/LABA/LAMA combinations. The challenge is to correctly define the characteristics that predict therapeutic success with each regime. We currently use clinical characteristics for this purpose, most importantly symptoms and exacerbation history. The future of COPD therapy is to expand this list, hopefully to include biomarkers such as blood eosinophil counts.
Acknowledgements
None.
Footnote
Conflicts of Interest: D Singh has received sponsorship to attend international meetings, honoraria for lecturing or attending advisory boards and research grants from various pharmaceutical companies including Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Genentech, GlaxoSmithKline, Glenmark, Merck, NAPP, Novartis, Pfizer, Respivert, Skypharma, Takeda, Teva, Therevance and Verona. And other author has no conflicts of interest to declare.
References
- Rodriguez-Roisin R. Toward a consensus definition for COPD exacerbations. Chest 2000;117:398S-401S. [Crossref] [PubMed]
- Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010;363:1128-38. [Crossref] [PubMed]
- Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347-65. [Crossref] [PubMed]
- Wedzicha JA, Calverley PM, Seemungal TA, et al. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med 2008;177:19-26. [Crossref] [PubMed]
- Singh D. New combination bronchodilators for chronic obstructive pulmonary disease: current evidence and future perspectives. Br J Clin Pharmacol 2015;79:695-708. [Crossref] [PubMed]
- Bateman ED, Ferguson GT, Barnes N, et al. Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur Respir J 2013;42:1484-94. [Crossref] [PubMed]
- Donohue JF, Maleki-Yazdi MR, Kilbride S, et al. Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respir Med 2013;107:1538-46. [Crossref] [PubMed]
- Celli B, Crater G, Kilbride S, et al. Once-daily umeclidinium/vilanterol 125/25 mcg in COPD: a randomized, controlled study. Chest 2014;145:981-91. [Crossref] [PubMed]
- Mahler DA, Decramer M, D'Urzo A, et al. Dual bronchodilation with QVA149 reduces patient-reported dyspnoea in COPD: the BLAZE study. Eur Respir J 2014;43:1599-609. [Crossref] [PubMed]
- Singh D, Gaga M, Schmidt O, et al. Effects of tiotropium + olodaterol versus tiotropium or placebo by COPD disease severity and previous treatment history in the OTEMTO® studies. Respir Res 2016;17:73. [Crossref] [PubMed]
- Bateman ED, Chapman KR, Singh D, et al. Aclidinium bromide and formoterol fumarate as a fixed-dose combination in COPD: pooled analysis of symptoms and exacerbations from two six-month, multicentre, randomised studies (ACLIFORM and AUGMENT). Respir Res 2015;16:92. [Crossref] [PubMed]
- Wedzicha JA, Decramer M, Ficker JH, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med 2013;1:199-209. [Crossref] [PubMed]
- Miravitlles M, Vogelmeier C, Roche N, et al. A review of national guidelines for management of COPD in Europe. Eur Respir J 2016;47:625-37. [Crossref] [PubMed]
- Vogelmeier CF, Bateman ED, Pallante J, et al. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group study. Lancet Respir Med 2013;1:51-60. [Crossref] [PubMed]
- Donohue JF, Worsley S, Zhu CQ, et al. Improvements in lung function with umeclidinium/vilanterol versus fluticasone propionate/salmeterol in patients with moderate-to-severe COPD and infrequent exacerbations. Respir Med 2015;109:870-81. [Crossref] [PubMed]
- Singh D, Worsley S, Zhu CQ, et al. Umeclidinium/vilanterol versus fluticasone propionate/salmeterol in COPD: a randomised trial. BMC Pulm Med 2015;15:91. [Crossref] [PubMed]
- Zhong N, Wang C, Zhou X, et al. LANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPD. Int J Chron Obstruct Pulmon Dis 2015;10:1015-26. [PubMed]
- Vogelmeier C, Paggiaro PL, Dorca J, et al. Efficacy and safety of aclidinium/formoterol versus salmeterol/fluticasone: a phase 3 COPD study. Eur Respir J 2016;48:1030-1039. [Crossref] [PubMed]
- Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-Glycopyrronium versus Salmeterol-Fluticasone for COPD. N Engl J Med 2016;374:2222-34. [Crossref] [PubMed]
- Anthonisen NR, Manfreda J, Warren CP, et al. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987;106:196-204. [Crossref] [PubMed]
- Siddiqui SH, Guasconi A, Vestbo J, et al. Blood Eosinophils: A Biomarker of Response to Extrafine Beclomethasone/Formoterol in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2015;192:523-5. [Crossref] [PubMed]
- Singh D, Papi A, Corradi M, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting β2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet 2016;388:963-73. [Crossref] [PubMed]
- Singh D, Roche N, Halpin D, et al. Current Controversies in the Pharmacological Treatment of Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2016;194:541-9. [Crossref] [PubMed]


