Development of a new interfacility extracorporeal membrane oxygenation transport program for pediatric lung transplantation evaluation
Introduction
Advances in the medical and surgical care of critically ill infants and children over the last several decades have resulted in the increased survival of patients with diseases which were once fatal in early life. However, this has in turn resulted in a growing population of children who now survive infancy, such as those with corrected congenital heart disease or cystic fibrosis, but then develop chronic illness and progressive organ failure related to their original disease process. For patients suffering end-stage failure of replaceable organs, transplantation may offer patients a renewed and improved quality of life. As pediatric organ transplant programs have proliferated over the years, more children now have the possibility of life-saving organ transplant, but access to such is limited to transplant centers.
Simultaneous with the increased population of children with end-stage organ failure has been the evolution of technologies and medical practice associated with advancement of extracorporeal membrane oxygenation (ECMO). ECMO offers intermediate-term, but nevertheless temporary, hemodynamic and/or respiratory support for patients with refractory hypoxic respiratory failure, septic shock, myocardial failure, or combinations thereof. Since ECMO carries the significant risk of serious complications (namely hemorrhage) and is resource-intensive, its use was originally intended for patients with undeniably severe yet reversible underlying illnesses. In earlier eras, the use of ECMO for the support of patients with known or suspected irreversible organ failure was thought inappropriate due to the significant risk of complications associated with ECMO without a meaningful endpoint. With the advent of pediatric transplant and the development of more reliable longer-term support devices (such as miniaturized ECMO circuits, centrifugal pumps, and ventricular assist devices) the prior resistance to use ECMO to support irreversible organ failure is increasingly being reconsidered. Use of ECMO to bridge patients to lung transplantation is increasingly being done at transplant centers (1-7). For these reasons, pediatric ECMO centers are finding themselves supporting patients with irreversible heart and/or lung failure for whom organ transplantation is a potentially life-saving intervention. Nationwide Children’s Hospital (NCH) in Columbus, Ohio, is a freestanding tertiary care pediatric referral center which offers ECMO-as-bridge to transplant for patients with irreversible heart or lung failure who are deemed eligible to receive an organ, based on a comprehensive consideration of comorbidities.
We report the de novo development of our institutional ECMO transport program and its use to bring ECMO-supported patients to NCH to undergo evaluation for heart or heart/lung transplantation. Our aim was to provide safe and effective ECMO management during transport from patients’ home institutions, where they had already been cannulated to ECMO by their treating physicians and who had subsequently been unable to be liberated from ECMO, to the NCH Heart Center Cardiothoracic intensive care unit (ICU) for evaluation for transplantation at our hospital. We sought to provide an opportunity for transplant to children who were either hospitalized at non-transplant centers, or who sought a second opinion from our transplant physicians after being deemed ineligible at their home institutions.
Methods
In 2012 the in-house NCH transport team met with the NCH ECMO team, cardiovascular perfusion, and the transplant program to discuss the development and deployment of a specialized ECMO transport team. The impetus to create the service was the presence of a known patient at an outlying hospital with irreversible lung failure and was not able to receive transplantation at her home hospital. Since the team’s inception, we have found transport of ECMO-supported patients for transplant evaluation to be an underserved niche, since most patients supported with ECMO who do not require a transplant are usually cared for at the nearest ECMO center. Currently, the NCH team does not offer cannulation to ECMO, only transport of already-cannulated ECMO patients.
Team
The NCH ECMO transport team composition was the first process to be completed. It was decided right away the standard NCH Transport team makeup would require modification to accomplish ECMO transport. In standard practice, the NCH team is composed of two trained transport clinicians (TCs) who provide care to transported patients plus a driver (or pilot). Medical care is provided by the TCs in conjunction with a medical control physician based at NCH. Medical control is usually provided by an accepting neonatologist, pediatric intensivist, or an emergency medicine physician depending on patient destination. It is not current practice for physicians to attend transports in person under standard conditions. For ECMO transport however, a specialized team composition was adapted to include accompanying ECMO specialists and a critical care physician. Early objectives were the delineation of roles and responsibilities.
Transport nurses
Patient care is delivered primarily by two experienced transport RNs. During ECMO transport, the RN duties include assessment and monitoring of patient status, vital signs, administration of medications, maintenance of relevant drains (e.g., chest tubes, etc.) and devices (mechanical ventilator, artificial airways), and documentation. The transport RNs are also responsible for assisting the physician when necessary for procedures or interventions. At the receiving unit, the RNs also provide standardized bedside handoff to receiving RNs and RRTs.
ECMO specialists (2/trip)
The ECMO providers include two practitioners who are either members of the NCH ECMO team (specially-trained RNs or RRTs with expertise and experience in ECMO delivery) or cardiovascular perfusionists. To date, all transports have included at least one perfusionist. The ECMO specialist/perfusionist responsibilities include management and maintenance of the ECMO machine, priming the transport ECMO circuit, transferring the patient to the transport circuit (when necessary), and titration of anticoagulation during transport under the direction of the accompanying physician.
Physician (1/trip)
An attending critical care physician accompanies ECMO transports for the entire trip. To date, pediatric intensivists have attended all our transports due to the ages of the patients; a neonatologist would accompany the transport of a neonate. Transport physicians are responsible for directing medical care during transport, ordering medications and interventions, communicating with referring physicians prior to transport, coordinating intratransport communications with the referring and receiving institutions, and counseling patients and families as to the goals, risks, and benefits of the transport itself.
In addition, crucial logistic support and coordination is provided by the clinical and administrative staffs of the transport team, the Heart Center at NCH, and the NCH Heart and Lung Transplant programs. These individuals secure insurance approval for transport, provide additional counsel to patients and families, and are typically the original point of contact for referring facilities.
Pre-transport consultation
An important aspect of the NCH ECMO transport program has been the development of pre-transport consultation to optimize vetting of potential patients. The process of transplant evaluation, listing, and peri-transplant care subject a patient and family to substantial upheaval including time away from home, family, and work. In addition, the transplant procedure itself incurs significant risk of complications in both the short and long term. The transport of a patient who is clearly ineligible to undergo an eventual transplant would be a significant disservice to all parties, and would incur unnecessary risk to the patient. However, transplant eligibility is not often easily determined without detailed review of the patient’s clinical course and additional investigation. When considering transporting a patient hundreds or even thousands of miles from home, our team felt it was necessary to develop a system to best identify suitable patients likely to benefit from transplant. As our center began to receive ECMO transport referrals, our transplant pulmonology team became increasingly facile with the process of remote evaluation of referred patients. This typically included a number of conversations with the referring physicians and the transfer of medical records and relevant images for review by the NCH-based teams. In certain cases in which the risk/benefit calculation was more difficult to assess from afar, the NCH transplant pulmonologist has traveled to the referring hospital to meet the patient and family in person and gather additional information from the local team. This allows for more detailed data gathering and allows families the opportunity to ask questions directly of the receiving team. In some cases on-site consultation has led to additional testing, such as follow-up echocardiography to assess right heart function in patients with long-standing hypoxic pulmonary vasoconstriction, which might not otherwise have been clinically indicated outside of a transplant evaluation but are useful in choosing optimal patients for transplant.
Pre-transport briefings
Once the pre-transport consultation process has identified a suitable referral the next step is pre-planning for transport. The ECMO and transport teams meet with the transplant team to discuss the specific details relevant to the transport. In addition to details of the patient’s clinical course, the pre-transport briefing focuses on: the patient’s ECMO cannulation configuration [venovenous (VV) or venoarterial ECMO]; presence of ECMO-related complications; current anticoagulation regimen; ECMO machinery and circuit type; mechanical ventilator mode and settings; airway devices; and level of sedation. The first briefing allows the team to begin preparing for the specific transport event and often prompts additional questions and data-gathering, so that ultimately there is a shared understanding of the patient and potential pitfalls during transport. After the initial briefing, specific members who will travel are selected and logistics of the transport are addressed, including scheduling the actual time for transport and contracting with aircraft providers if an airplane will be necessary (helicopter and ground transport is accomplished with the NCH transport team’s own vehicles). Contingency plans are made including alternative landing sites in the event of mechanical problems with aircraft. Equipment lists and checklists are reviewed. Within 24 hours of the trip the transport physician contacts the referring team to discuss the patient’s current status, significant recent events, and to gather updated clinical and laboratory data. Equipment and supplies are gathered, blood products are requested of the referring institution’s medical team, an electronic medical record is generated by the NCH Admitting Department, and final departure times are set.
Post-transport debriefing
As soon as possible after the completed transport, typically the next weekday, the team members meet with the transport team medical directors and administrators, ECMO team leadership, and the transplant physicians to discuss the trip in detail. All aspects are reviewed including unexpected events (if any) the effectiveness of pre-transport preparation, equipment and team performance, and effectiveness of communication. The post-transport debriefing allows the involved teams and services to refine processes, equipment lists and checklists, and to further define roles and responsibilities. Action items are generated based on needs identified in the post-transport phase.
Results
Patients
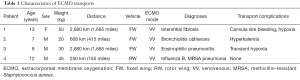
Table 1 summarizes characteristics of our ECMO transport patients. To date the NCH ECMO transport team has transported four patients from three institutions to the Heart Center Cardiothoracic ICU for evaluation for transplant. Transport of patient 1 has been reported previously (8). Patients 1 and 3 were each transported 1,665 miles from the same referring hospital. Patient 2 was transported 415 miles. Patients 1, 2, and 3 were transported via fixed wing (FW) aircraft using an ambulance for transport to and from the relevant airports. Patient 4 was transported 155 miles directly from hospital to hospital via EC-145 helicopter which required customization of equipment and has been previously reported by our group (9) (Figure 1). All patients had been cannulated to VV ECMO at their home institutions with the intention of using ECMO as a bridge to recovery. Each patient had failed multiple attempts of lung recruitment and ECMO weaning prior to the patients’ physicians considering referral for transplant evaluation. Surprisingly, none of the patients had cystic fibrosis or end-stage heart failure secondary to primary cardiac disease. Two patients, an 8 years old boy (patient 3) and a 12 years old boy (patient 4) were previously healthy prior to the onset of acute severe hypoxic respiratory failure requiring ECMO. Patients 1 and 2 had longstanding respiratory disease with acute decompensation and progression of their underlying illness at the time of ECMO cannulation. Additional referrals have been received which have ultimately not been transported, due to either improvement at their home institution or death prior to transport.
Full table
No intra-transport mortality or significant morbidity has occurred. Patient 1 demonstrated cannula site bleeding necessitating transfusion of red cells during transport. Patient 1 also developed hypoxia during flight which was resistant to manipulations of the ventilator and required reduced altitude of the aircraft. Patient 2 required a period of reduced ECMO flow after switching to the transport circuit due to high potassium concentration in the prime blood of the new circuit. He manifested transient hypoxia as his flow was slowly increased and also required calcium chloride and sodium bicarbonate due to widened QRS complexes after exposure to the new primed circuit. After re-establishing full ECMO flow and the above therapies for hyperkalemia, hypoxia and QRS abnormalities resolved. Patient 3 developed desaturation after transfer from the transport cot which persisted despite manipulation of both ECMO blood flow and sweep gas oxygen concentration. To prevent worsened hypoxia during transport, the decision was made to pressurize the aircraft cabin at ground level prior to takeoff and fly at lower altitude. Ultimately, this patient’s saturations markedly improved after transfer to the airplane and repositioning. In retrospect we suspected, but were unable to prove, that his desaturation was likely the result of a temporary malposition of the ECMO cannula (a bicaval dual-lumen VV cannula), caused by body position changes during transfer to the cot which led to increased recirculation. Patient 4 experienced no unexpected events during his helicopter transport. No complications related to malfunction of ECMO equipment, ventilators, monitors, medication delivery systems, or vehicle mechanical problems occurred in any patient. Aside from the suspected temporary malposition of an ECMO cannula in patient 3, no other cannula-related complications occurred and no devices were dislodged. Only patient 2, who had already experienced difficulties with anxiety throughout his hospitalization, required significantly increased sedation to accomplish transport. The other patients remained on their baseline doses of sedatives and were in general alert and cooperative during travel. Unfortunately, the substantial restrictions on weight and physical space relative to the size of the team and equipment have prevented a parent or guardian from accompanying the transport in all cases except patient 2.
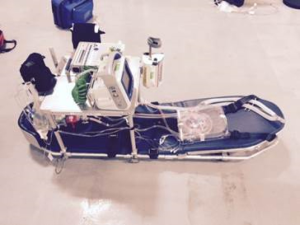
Although the range of the EC-145 helicopter is limited compared to fixed-wing airplanes, the ECMO transport team noted a preference for this vehicle when feasible. Helicopter transport obviates transfers into and out of an ambulance, both at the sending and receiving airports, which are otherwise needed during FW transport. The helicopter is thus associated with fewer episodes of patient transfer and avoids opportunities for dislodgement or malposition of devices. Using NCH’s dedicated transport helicopter also allows from much quicker response times and coordination of transport since arrangements with non-dedicated air transport providers are unnecessary. Additionally the helicopter’s rear-opening loading doors allows for much easier loading of the patient into the aircraft relative to the airplane in which the patient orientation must be rotated to align with the long axis of the plane once the patient is passed through the door (Figure 2).
Procedure development
Through the debriefing process several procedures have been modified or developed to optimize ECMO transport. The earliest refinement was the adoption of the CardiohelpTM (MAQUET GmbH, Rastatt, Germany) ECMO pump as our ECMO transport machine of choice. The use of this miniature portable ECMO machine was made necessary by the significantly limited physical space in the aircraft used for transport. Previous authors have reported use of military aircraft with associated larger space for equipment and crew (10). The Hawker jets and EC-145 helicopters used by our team are more confined. The CardiohelpTM system combines the circuit, oxygenator, and centrifugal pump impeller into one integrated piece which is installed onto the drive unit. A single display screen can be toggled through different views depending on the data being sought. The resulting system is exceptionally small and light, making it an ideal transport machine. For patients already supported with a CardiohelpTM at their home institution (patient 4 in our series), the integrated circuit/oxygenator unit is simply switched to the NCH team’s drive unit. For situations in which other ECMO pumps are in use a circuit change is done: the transport circuit is de-aired and primed crystalloid while en route to the referring hospital and blood-primed at the bedside. The patient is removed from their circuit and new airless connections are made to the NCH team’s blood-primed CardiohelpTM. The transfer to the new circuit is routinely finished in less than one minute by our team. By arrangement with the referring team’s physicians, nurses and respiratory therapists, during the brief period of time the patient is off ECMO to accomplish the transition to NCH equipment, the referring physicians manage any patient decompensation while NCH team members concentrate on managing the circuit change and the NCH ECMO machine.
In addition to the adoption of single transport ECMO device and processes for transfer to this circuit, we have developed and refined forms for patient data collection, equipment lists, and checklists. These tools were developed to ensure safe and effective management of high-stakes transport scenarios which are rare. A systems-based patient data sheet was generated to allow for standardized handoff between centers in order to avoid medical errors related to communication lapses. The ECMO transport equipment list was generated and subsequently refined to balance the desire for a complete set of supplies with backup pieces against substantial restrictions on equipment weight and space. A pre-departure checklist was created to address the potential effects of altitude on the patient and his/her medical devices. For example, filling the endotracheal tube cuff with saline instead of air avoids expansion and potential rupture of the cuff as the airplane ascends. This checklist is reviewed just prior to leaving the patient’s hospital bed space. These tools are considered “living documents” and are subject to regular review and revision as part of the post-transport debriefing process.
Discussion
In this report we describe a multidisciplinary undertaking to develop a de novo program to provide interfacility transport of ECMO-supported patients for the purpose of evaluation for lung transplant at a specialized center. Although the NCH ECMO transport team is able and willing to transport ECMO-supported patients to NCH for whatever cause, the inspiration for the team’s genesis was our transplant pulmonologist’s knowledge of the existence of children with irreversible lung disease in distant locations who were unlikely to undergo an evaluation for potentially life-saving lung transplant. For most patients, the prohibiting factor is the lack of a pediatric lung transplant center in their region. The vast majority of states in the US (44 of them) have no pediatric transplant center, and all such centers are clustered in academic referral centers, usually in large cities (11). This geographic dissonance leaves many patients far from the nearest transplant program when the irreversibility of their lung disease becomes apparent. Our team was designed to increase access to transplant evaluation for patients cannulated to ECMO, but subsequently unable to be weaned.
In other circumstances transplant has been precluded by the historically poor outcomes of pediatric transplant patients who require ECMO support at the time of their transplant operation. The state of literature continues to consist primarily of small case series (4,7) but nevertheless, high mortality and increased incidence of significant complications (6) were important features of earlier experience in pediatric ECMO patients receiving lung transplantation. For these reasons, some pediatric transplant programs do not list patients who require ECMO support for transplant until they can be weaned off of ECMO. Although it is impossible to refute this approach outright, more recent analyses of data sets with propensity score matching indicate that survival for children on ECMO at the time of transplant may not be significantly different than those who do not require ECMO. Moreover, the earlier literature characterized by poor outcomes predates the development of centrifugal pumps, miniaturized ECMO circuits, and general movement from venoarterial to VV ECMO configurations which are all potentially associated with fewer ECMO related complications(1,3,5,7). NCH offers ECMO as bridge to transplant for local patients, most of whom have cystic fibrosis, when critical illness occurs in previously-listed patients. The NCH ECMO transport team has expanded this service to other critically ill patients who may be located in transplant centers not offering transplant to ECMO-supported patients.
Regardless of the impediment to a patient undergoing transplant evaluation at their home institution, an important responsibility of the receiving NCH teams is the clear communication of goals and expectations. An invaluable principle of the pre-transport consultation process had been the discussion between the NCH-based transplant pulmonology team and the patient and his/her family during which the goals of the transport are made clear. Patients and families are counseled that transport is being offered to facilitate a thorough evaluation to determine eligibility for potential listing for transplant and the need to get the patient physically relocated to NCH where such an operation would be performed. No guarantee of undergoing a transplant operation, or even listing for an organ, is made.
Interfacility transport of ECMO-supported patients has been described in several published series over the last couple decades (10,12,13). In general ECMO transport is associated with low rates of complications and very low rates of significant complications. Most authors report no deaths or serious events associated with transport series of several dozens of missions. In almost all published series, outcomes of ECMO-transported patients are not dissimilar to in-house (receiving facility) ECMO patients and are in line with aggregate survival rates from the Extracorporeal Life Support Organization international registry. The teams detailed in these reports resemble our own in several aspects: a multidisciplinary team with physician attendance and specially-trained ECMO personnel, modification of equipment to accommodate space and weight restrictions, and the ability to transport using various vehicles. Our team differs somewhat from others in that it was designed and implemented after newer circuits and pumps became available. Centrifugal pumps, the CardiohelpTM machine, simplified circuits, and bicaval dual lumen VV cannula were unavailable at the inception of ECMO transport in the mid-1980s and more teams have written of their experiences using these innovations as they have come to the market. Our team, being only active since 2012, has taken full advantage of newer componentry. Nevertheless, we have made our own contributions to the field by customizing standard transport equipment to allow rotor wing (RW) ECMO transport using the EC-145 helicopter (9). Previous authors have used the larger Sikorsky-76 helicopter (13) which can accommodate larger loads. The customized sled developed by our team allows for use of the NCH transport team’s dedicated helicopter with FAA-compliant equipment (Figure 2).
The CardiohelpTM machine has facilitated our team’s development with implications that are worthy of mention. Since its release, centers using this miniaturized ECMO machine have produced a body of literature detailing its use in the transport setting (14-17). In our experience the CardiohelpTM has performed admirably with no mechanical complications to date. As with all non-occlusive centrifugal pump systems, the CardiohelpTM circulates blood by creation of a constrained vortex. Such systems are sensitive to preload and afterload but we experienced no meaningful change in blood flow through the ECMO circuit during the significant accelerations and decelerations of takeoff and landing during our FW transports. Reductions in flow were fleeting and not associated with changes in oxygen saturations in our VV ECMO patients. However, our institutional preference for this machine obligates patients not using it at their home institution to undergo a circuit change and associated complications which would not otherwise be necessary. (The experience with patient 2, who developed ECG changes symptomatic of hyperkalemia when transitioned to our circuit with high potassium content from prime blood illustrates this risk.) Although we have transported one patient using another pump and circuit by airplane (8) our helicopter-ready sled configuration requires a CardiohelpTM to fit in the EC-145 (9).
Many ECMO transport teams send personnel to cannulate patients at the referring hospital prior to transport back, meaning the patient’s ECMO course begins almost immediately preceding departure (10,12,13,18,19). Such teams provide ECMO services to critically ill patients too ill to be transported conventionally to an ECMO center. Our niche is different in that we have transported patients supported at ECMO centers, often for many weeks, to NCH for transplant evaluation. Had they not needed transplant, there would be very little reason for these children to have left their home hospital. The decision to leave an ICU to which the child and family have become accustomed, and with whom the therapeutic alliance is strong, in order to travel far from home for a high-risk operation (maybe) is an incredible burden for patients and families to undertake. These considerations notwithstanding, the use of ECMO transport to provide access to transplant evaluation has, in our limited experience, proven feasible and safe. Such services have the potential to offer life-saving interventions to patients who otherwise have extremely limited potential for good outcome.
Acknowledgements
The authors are indebted to Don Hayes Jr, MD, Philip L. Holt, BSN, CMTE and Thomas J. Preston, CCP for inspiring the development of this new transport service and for their tireless efforts on behalf of the team and its patients. We gratefully acknowledge the patients and their families for their trust.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Fuehner T, Kuehn C, Hadem J, et al. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am J Respir Crit Care Med 2012;185:763-8. [Crossref] [PubMed]
- Hayes D Jr, McConnell PI, Tobias JD, et al. Survival in children on extracorporeal membrane oxygenation at the time of lung transplantation. Pediatr Transplant 2015;19:87-93. [Crossref] [PubMed]
- Hoopes CW, Kukreja J, Golden J, et al. Extracorporeal membrane oxygenation as a bridge to pulmonary transplantation. J Thorac Cardiovasc Surg 2013;145:862-7; discussion 867-8. [Crossref] [PubMed]
- Jackson A, Cropper J, Pye R, et al. Use of extracorporeal membrane oxygenation as a bridge to primary lung transplant: 3 consecutive, successful cases and a review of the literature. J Heart Lung Transplant 2008;27:348-52. [Crossref] [PubMed]
- Olsson KM, Simon A, Strueber M, et al. Extracorporeal membrane oxygenation in nonintubated patients as bridge to lung transplantation. Am J Transplant 2010;10:2173-8. [Crossref] [PubMed]
- Puri V, Epstein D, Raithel SC, et al. Extracorporeal membrane oxygenation in pediatric lung transplantation. J Thorac Cardiovasc Surg 2010;140:427-32. [Crossref] [PubMed]
- Turner DA, Cheifetz IM, Rehder KJ, et al. Active rehabilitation and physical therapy during extracorporeal membrane oxygenation while awaiting lung transplantation: a practical approach. Crit Care Med 2011;39:2593-8. [Crossref] [PubMed]
- Hayes D Jr, Galantowicz M, Preston TJ, et al. Cross-country transfer between two children's hospitals of a child using ambulatory extracorporeal membrane oxygenation for bridge to lung transplant. Pediatr Transplant 2013;17:E117-8. [Crossref] [PubMed]
- Holt PL, Hodge AB, Ratliff T, et al. Pediatric Extracorporeal Membrane Oxygenation Transport by EC-145 With a Custom-Built Sled. Air Med J 2016;35:171-5. [Crossref] [PubMed]
- Coppola CP, Tyree M, Larry K, et al. A 22-year experience in global transport extracorporeal membrane oxygenation. J Pediatr Surg 2008;43:46-52; discussion 52. [Crossref] [PubMed]
- Hayes D Jr, Preston TJ, Galantowicz M, et al. Improving accessibility to lung transplantation for children through air transport. Air Med J 2015;34:52-3. [Crossref] [PubMed]
- Bryner B, Cooley E, Copenhaver W, et al. Two decades' experience with interfacility transport on extracorporeal membrane oxygenation. Ann Thorac Surg 2014;98:1363-70. [Crossref] [PubMed]
- Cabrera AG, Prodhan P, Cleves MA, et al. Interhospital transport of children requiring extracorporeal membrane oxygenation support for cardiac dysfunction. Congenit Heart Dis 2011;6:202-8. [Crossref] [PubMed]
- Alwardt CM, Wilson DS, Alore ML, et al. Performance and Safety of an Integrated Portable Extracorporeal Life Support System for Adults. J Extra Corpor Technol 2015;47:38-43. [PubMed]
- Arlt M, Philipp A, Voelkel S, et al. Hand-held minimised extracorporeal membrane oxygenation: a new bridge to recovery in patients with out-of-centre cardiogenic shock. Eur J Cardiothorac Surg 2011;40:689-94. [PubMed]
- Haneya A, Philipp A, Foltan M, et al. First experience with the new portable extracorporeal membrane oxygenation system Cardiohelp for severe respiratory failure in adults. Perfusion 2012;27:150-5. [Crossref] [PubMed]
- Philipp A, Arlt M, Amann M, et al. First experience with the ultra compact mobile extracorporeal membrane oxygenation system Cardiohelp in interhospital transport. Interact Cardiovasc Thorac Surg 2011;12:978-81. [Crossref] [PubMed]
- Isgrò S, Patroniti N, Bombino M, et al. Extracorporeal membrane oxygenation for interhospital transfer of severe acute respiratory distress syndrome patients: 5-year experience. Int J Artif Organs 2011;34:1052-60. [Crossref] [PubMed]
- Lucchini A, De Felippis C, Elli S, et al. Mobile ECMO team for inter-hospital transportation of patients with ARDS: a retrospective case series. Heart Lung Vessel 2014;6:262-73. [PubMed]



