The case for preoperative aspirin administration in patients undergoing elective CABG: is it open or closed?
Aspirin is one of the most widely used and commonly prescribed medications in the world. It was first approved by the US Food and Drug Administration (FDA) in 1985 for the secondary prevention of cardiovascular events in patients following an acute myocardial infarction (MI) (1) and continues to be prescribed widely for patients with and those at heightened risk for atherosclerotic cardiovascular disease (ACVD)-related events.
Aspirin inhibits the synthesis of platelet thromboxane A2 and reduces platelet assembly and aggregation at sites of vascular injury (2). Thromboxane A2 is the end-product of an enzymatic conversion that begins with arachidonic acid being converted to prostaglandin H2 by cyclooxygenases (COX1 & COX2). Only COX1 is found within platelets. These prostaglandins are subsequently converted by specific synthases to down-stream prostaglandins, including prostaglandin I2, E2, D2, and F2α and thromboxane A2 (3). Aspirin irreversibly inhibits the binding of arachidonic acid to COX1 and subsequently prevents the formation of thromboxane A2. Since platelets cannot regenerate COX1, the resumption of platelet activity depends on bone marrow production of new platelets (4).
Aspirin has been studied extensively in the treatment of patients with acute coronary syndrome (ACS) (5,6), yielding a reduction in death and nonfatal MI. A large and widely cited meta-analysis of aspirin use for secondary prevention in patients with a history of MI, stroke, transient ischemic attack or other high risk conditions (unstable angina, stable angina, peripheral vascular disease) was conducted by the Antiplatelet Trialists’ Collaborators. They identified a significant reduction of non-fatal MI (one third reduction) and vascular death (one sixth) among these high risk patients (7). Accordingly, aspirin has become a mainstay in the treatment of patients with established ACVD.
What is the current position on preoperative aspirin administration?
Because patients with ACVD on aspirin therapy may at times require cardiac or non-cardiac surgery, decisions surrounding antiplatelet medications in the perioperative period have been emphasized in several contemporary management guidelines. These guidelines are summarized in Table 1. Considered collectively, a consistent message for preoperative aspirin administration among patients undergoing CABG is lacking. Does this represent true equipoise or a lack of randomized clinical trial-based evidence?
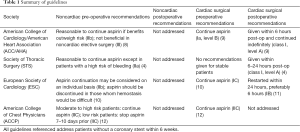
Full table
What is the evidence?
Myles and colleagues recently conducted a much-needed and thought-provoking study, “Stopping vs. Continuing Aspirin before Coronary Artery Surgery-(ATACAS)” (13)—a randomized trial of 27 patients scheduled for elective CABG, but who were considered to have increased risk for cardiovascular and other serious post-operative events based on either age >70 years, left ventricular impairment, concomitant valvular or aortic surgery, redo-cardiac surgery, chronic obstructive lung disease (COPD), renal impairment, obesity, pulmonary hypertension, or peripheral vascular disease. Aspirin taken within 4 days of surgery was an exclusion. Eligible patients were then randomized, employing a 2-by-2 factorial design, to aspirin versus placebo and tranexamic acid versus placebo. Patients received either 100 mg enteric-coated aspirin or placebo within 1 to 2 hours of surgery. Patients received postoperative aspirin according to the practices at the participating hospitals. The median time to postoperative aspirin in the aspirin group was 18.5 hours (interquartile range, 12.3–22.9) and was 18.8 hours (interquartile range, 13.1–23.5) in the placebo group. The primary outcome was a composite of death and thrombotic events (including nonfatal MI). MI was defined as a rise of troponin or creatine kinase (CK)-MB plus as least one of the following: ischemic symptoms, development of pathological Q waves on a surface 12-lead electrocardiogram (ECG), or ST segment elevation or depression; or a significant rise in troponin I (>10 ng/mL), troponin T >4.0, or CK-MB >3 times the upper limit of normal in patients with no Q waves. Secondary outcomes included death, nonfatal MI, major hemorrhage (requiring surgical exploration), cardiac tamponade, and need for transfusion. Postoperative antiplatelet medication was managed according to local hospital practice.
Overall, there were no significant differences between study groups for either the primary or secondary outcome measures. A primary outcome occurred in 202 (19.3%) of aspirin-treated patients versus 215 (20.4%) in the placebo group [risk ratio: 0.94; 95% confidence interval (CI): 0.80–1.12; P=0.55]. Reoperation for hemorrhage occurred in 19 (1.8%) patients in the aspirin group and 22 (2.1%) patients in the placebo group (risk ratio: 0.87; 95% CI: 0.47–1.60; P=0.75). There was not a significant difference in blood product transfusion rates between groups. The investigators concluded that administrating aspirin preoperatively did not increase the risk of bleeding nor decrease the risk of death or MI. The findings from the tranexamic acid arm of the study have not yet been published.
The ATACAS study represents the largest randomized clinical trial performed to date that was designed to answer a question encountered regularly in clinical practice. Accordingly, the investigators and participating sites should be acknowledged for their meaningful contribution; however, we believe that it is important for clinicians to better understand the patients and conditions, also common in daily patient care, not represented in the study, as well as several potential limitations that collectively lead us to conclude that there are opportunities for future research undertakings i.e., the case is open.
What was the primary take-home message?
The overall findings of ATACAS suggest that aspirin, administered within 1–2 hours of elective CABG, neither reduces the incidence of post-operative MI, stroke or death nor increases the risk of major bleeding or blood product transfusion, in stable patients.
Was the chosen primary endpoint optimal?
The primary safety and efficacy endpoints of ATACAS, considered collectively, represent many of the post-operative events that impact patient outcomes, length of hospital stay, resource utilization and cost. The missing endpoint that many cardiologists and cardiac surgeons would find important is early (within the first 30 days) saphenous vein occlusion (14). Vein graft harvesting causes endothelial disruption (injury) and localized platelet activation. The primary endpoint of MI would likely capture some of these events, but many could have been missed due to “silent” graft occlusion—i.e., no ECG changes or cardiac enzyme elevations. While the study would have been more complex and costly if routine coronary angiography or cardiac computed tomography (CT) angiography was included, the early equivalent of incomplete revascularization is known to be associated with less favorable clinical outcomes (15).
Was the preparation and timing of aspirin administration prior to CABG optimal?
The investigators chose to use an enteric-coated aspirin preparation. In settings where a rapid onset of platelet inhibition is required, non-enteric coated aspirin represent the standard of care. A study evaluating the pharmacokinetics of enteric versus non-enteric coated aspirin given orally at a dose of 325 mg to healthy subjects showed that it took 30 minutes for maximum thromboxane A2 inhibition with the non-enteric coated preparation compared to 240 minutes for the enteric coated preparation. The thromboxane activity decreased by only 20% within 30 minutes with the enteric coated tablet, which is not sufficient to prevent thromboxane-related platelet aggregation (16). Patients participating in the “Continuing vs. Stopping Aspirin” study were given a 100 mg enteric coated aspirin 1–2 hours prior to surgery. Based on the well-known pharmacokinetics of aspirin, there is a high likelihood that there was not sufficient time to reach Cmax and maximum platelet inhibition prior to the start of the surgery. Once surgery begins and the patient is placed on cardiopulmonary bypass, local concentrations and related pharmacodynamics effects i.e., at coronary sites of plaque of newly placed bypass conduits would be quite low.
At least one study evaluating the bioequivalence of different aspirin formulations found that enteric coated aspirin had a higher rate of incomplete thromboxane inhibition compared to dispersible aspirin and an even higher rate of attenuated effects among subjects of high body weight (17). In the study by Myles and colleagues, the mean weight in the aspirin group was 85 kg, which, extrapolating to the work of Cox cited above, corresponds to a 20–30% probability of suboptimal (sub-threshold) platelet inhibition.
Was it wise to stop aspirin prior to CABG?
This study had a higher than expected rate of MI in both groups. The ATACAS investigators speculate that this was the end-result of closer monitoring and increased troponin surveillance i.e., higher detection rate. Could this actually be because aspirin was stopped in many patients at least 4 days prior to surgery, leading to a higher risk of complications? By stopping aspirin early, there could have also been patients who were excluded from participating in the study if they experienced a coronary event in the interim before CABG. This would have biased the outcomes. Perhaps there is a separate take-home message that applies to some high-risk patients- aspirin should not be stopped prior to CABG.
What is the optimal approach to aspirin administration in high-risk patients?
Where does this leave us in terms of treatment and management in the future? Most of the guidelines do not advocate stopping aspirin in ACS, which was not the focus of ATACAS. A previously conducted cohort study found that disruption of antiplatelet therapy portended worse outcomes-particularly in patients with ACS when compared to nonusers and patients remaining consistently on antiplatelet therapy (18). In ATACAS preoperative aspirin administration was not associated with a greater risk of major bleeding and blood product use. Does this support the hypothesis that aspirin given 1–2 hours before placing a patient on cardiopulmonary bypass does not achieve a platelet-inhibiting effect?
What are the best next steps?
It will be interesting to review the data from the tranexamic acid arm of ATACAS; however, given the lack of either a bleeding signal or reduction in clinical thrombotic and other major post-operative events it may be challenging to interpret and translate the findings with respect to preoperative aspirin use. Additional randomized clinical trials employing a strategy of non-enteric coated aspirin given ~4–6 hours prior to CABG may be instructive. Large-scale registries and pragmatic trials of patients undergoing CABG either on aspirin or not on aspirin may also provide complementary and hypothesis-generating observations.
Aspirin is a mainstay in the management of patients with ACVD. The findings from ATACAS suggest that aspirin, given 1–2 hours before elective CABG is neither beneficial nor harmful. Clinicians must always use sound judgement in patient care, prescribing aspirin to patients who stand to benefit from its antithrombotic effects and those in whom the risk outweighs the benefit. Though not tested in ATACAS, we recommend continuing aspirin in patients with ACS up to the time of surgery. In addition, all patients undergoing CABG should receive aspirin post-operatively-typically within 6–12 hours of surgery unless hemostasis has not been successfully achieved. Although a century in the making, aspirin and its optimal use in clinical practice remains somewhat of a mystery—the case is far from closed.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Provenance: This is a Guest Editorial commissioned by Section Editor Busheng Zhang, MD, PhD (Department of Cardiac Surgery, Shanghai Chest Hospital, Shanghai Jiaotong University, Shanghai, China).
References
- Aspirin for heart patients. FDA Drug Bull 1985;15:34-6. [PubMed]
- Sangkuhl K, Shuldiner AR, Klein TE, et al. Platelet aggregation pathway. Pharmacogenet Genomics 2011;21:516-21. [Crossref] [PubMed]
- Awtry EH, Loscalzo J. Aspirin. Circulation 2000;101:1206-18. [Crossref] [PubMed]
- Ferraris VA, Saha SP, Oestreich JH, et al. 2012 update to the Society of Thoracic Surgeons guideline on use of antiplatelet drugs in patients having cardiac and noncardiac operations. Ann Thorac Surg 2012;94:1761-81. [Crossref] [PubMed]
- Baigent C, Collins R, Appleby P, et al. ISIS-2: 10 year survival among patients with suspected acute myocardial infarction in randomised comparison of intravenous streptokinase, oral aspirin, both, or neither. The ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. BMJ 1998;316:1337-43. [Crossref] [PubMed]
- Nyman I, Larsson H, Wallentin L. Prevention of serious cardiac events by low-dose aspirin in patients with silent myocardial ischaemia. The Research Group on Instability in Coronary Artery Disease in Southeast Sweden. Lancet 1992;340:497-501. [Crossref] [PubMed]
- Collaborative overview of randomised trials of antiplatelet therapy--I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists' Collaboration. BMJ 1994;308:81-106. [Crossref] [PubMed]
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014;64:e77-137. [Crossref] [PubMed]
- Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol 2011;58:e123-210. [Crossref] [PubMed]
- Kristensen SD, Knuuti J. New ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management. Eur Heart J 2014;35:2344-5. [Crossref] [PubMed]
- Sousa-Uva M, Storey R, Huber K, et al. Expert position paper on the management of antiplatelet therapy in patients undergoing coronary artery bypass graft surgery. Eur Heart J 2014;35:1510-4. [Crossref] [PubMed]
- Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e326S-50S.
- Myles PS, Smith JA, Forbes A, et al. Stopping vs. Continuing Aspirin before Coronary Artery Surgery. N Engl J Med 2016;374:728-37. [Crossref] [PubMed]
- Chesebro JH, Lam JY, Fuster V. The pathogenesis and prevention of aortocoronary vein bypass graft occlusion and restenosis after arterial angioplasty: role of vascular injury and platelet thrombus deposition. J Am Coll Cardiol 1986;8:57B-66B. [Crossref] [PubMed]
- Guerra M, Mota JC. Impact of incomplete surgical revascularization on survival. Interact Cardiovasc Thorac Surg 2012;14:176-82. [Crossref] [PubMed]
- Cerletti C, Marchi S, Lauri D, et al. Pharmacokinetics of enteric-coated aspirin and inhibition of platelet thromboxane A2 and vascular prostacyclin generation in humans. Clin Pharmacol Ther 1987;42:175-80. [Crossref] [PubMed]
- Cox D, Maree AO, Dooley M, et al. Effect of enteric coating on antiplatelet activity of low-dose aspirin in healthy volunteers. Stroke 2006;37:2153-8. [Crossref] [PubMed]
- Collet JP, Montalescot G, Blanchet B, et al. Impact of prior use or recent withdrawal of oral antiplatelet agents on acute coronary syndromes. Circulation 2004;110:2361-7. [Crossref] [PubMed]


