Review: capsule colonoscopy—a concise clinical overview of current status
Colon capsule specifications
The first-generation colon capsule endoscopy (CCE) was introduced in 2007 (1). The main difference between the CCE and conventional small bowel capsule endoscopes was the introduction of two optical domes at either end of the capsule to enable fuller visualisation of the relatively wider lumen structure in the colon. The currently available second-generation CCE (CCE-2) (Medtronic, Minneapolis, USA) (Figure 1) consists of a swallowable video capsule (11.6 mm × 31.5 mm, weight 2.9 g), which has an improved optical system allowing for nearly 360° coverage via two 172° angle cameras. The battery life is about 10 hours. It is equipped with the adaptive frame rate function, which modulates the frame rate according to capsule progression speed in order to save battery and optimise video length. The frame rate alternates between 4–35 images per second depending on the motion of the capsule. The RAPID® reviewing system allows dual communication between the CE and data recorder. In addition, the new data recorder is able to actively elaborate information received from the capsule and to alert the patient at planned intervals to drive the laxative booster ingestion (2,3). At present the main clinical indications for CCE are: (I) completion of incomplete colonoscopy (Figure 2); (II) polyp detection (Figure 3); and (III) investigation of inflammatory bowel disease (IBD) (Figure 4).


The need for complete colonoscopy
Conventional colonoscopy is the gold standard in bowel cancer screening, but remains an uncomfortable experience for many patients, and clinical performance varies widely between endoscopists and centres (4-6). As the incidence of bowel cancer increases, there is extra demand for high quality colonoscopy services. Therefore, a working group was formed in 2013 from the Joint Advisory Group on Gastrointestinal Endoscopy (JAG), the British Society of Gastroenterology (BSG), and the Association of Coloproctology of Great Britain and Ireland (ACPGBI), to review existing and define new quality assurance measures and key performance indicators in colonoscopy (7).
The major key quality indicators are caecal intubation rate and adenoma detection rate. Nowadays, caecal intubation rate is a well-recognized measure of colonoscopy quality and the working group has defined a target rate of 95%. While large scale screening colonoscopy studies have reported a completion rate above this recommended threshold (8-10), population-based studies report that the caecal intubation rate in clinical practice is far less (approximately 80–85%) (11-13).
Incomplete colonoscopy is associated with missed lesions (14). Imperiale et al. (15) calculated that up to 50% of clinically significant lesions would be missed by failing to visualise the entire colon. Consistently, a study by Brenner et al. (16) showed a 2-fold increased risk in proximal cancer after incomplete colonoscopy. Recently, Ridolfi et al. (17) reported 171 patients with initial incomplete colonoscopy. In 21 patients (12%) undergoing follow-up examinations, significant lesions were discovered by either repeating colonoscopy (80 patients) or radiological imaging tests (91 patients). Stoffel et al. (18) conducted a study on post-colonoscopy colorectal cancer (CRC). They found that in patients diagnosed with CRC within a year after colonoscopy, 38% had had incomplete colonoscopies compared to 16% of those who were diagnosed 1–10 years after colonoscopy. The same study reported that tumours found in patients who had had colonoscopies were more likely to be proximal; this could have been why these tumours had been missed initially. le Clercq et al. (19) reported on a cohort of patients diagnosed with metachronous colorectal cancer diagnosed more than 6 months after initial diagnosis of a primary CRC. They found that 43% of metachronous CRC were attributable to non-compliance with surveillance, 43% to missed lesions and 3.2% to inadequate examination.
Factors associated with incomplete colonoscopy include poor bowel preparation, severe diverticulosis or stenosis, tortuous and redundant colon, low body mass index, previous abdominal surgery, female sex, young age, patient intolerance and ineffective sedation (11-13,20-24). Therefore, several technical and technological solutions have been suggested in recent years to achieve complete colonoscopy in these situations. These include the use of optimized bowel prep schedules or imaging techniques (i.e., magnetic scope guidance or fluoroscopy) to monitor the scope progression, use of deeper sedation protocols, of water immersion technique or carbon dioxide insufflation, or the use of specific endoscopes (i.e., stiffer or thinner endoscopes, device-assisted endoscopes). Moreover, the endoscopist’s manual dexterity and expertise significantly affect the caecal intubation rate (25,26). Therefore, the large majority of patients with initial incomplete colonoscopy can undergo a successful repeated colonoscopy at tertiary referral centers (22,23,25,26), where expert endoscopists and dedicated endoscopes are both available. Nevertheless, in case of initial incomplete colonoscopy, several techniques alternative to conventional colonoscopy, such as computer tomography colonography (CTC) or CCE are also recommended. There appears to be a low to minimal risk of CCE retention (2,3,27).
Use of CCE to complete colonoscopy
In 2008 Spada et al. (1) reported the first case where CCE managed complete colon inspection where conventional colonoscopy had been stopped at the sigmoid by inflammatory stenosis. In this patient the capsule showed a 10 mm polyp not reached on conventional colonoscopy. Thereafter, other case-reports (28) and small case-series (29) have reported successful complete colon inspection by CCE in patients with previous incomplete conventional colonoscopy. To the best of our knowledge, six cohort studies (30-34) have been published so far on the use of CCE to complete colon examination (Table 1). These studies have collected more than 450 patients overall, with a completion rate of approximately 90% of cases (range, 72–98%). Significant findings were identified in more than one third of patients (range, 23–49%). Based on these data, in 2012 the ESGE issued a guideline (37) recognising CCE as a feasible and safe tool for visualization of the colonic mucosa in patients with incomplete colonoscopy without stenosis. In the same paper the authors recommended further randomized trials comparing CCE with radiological imaging and/or conventional colonoscopy in order to confirm the efficacy of CCE in this setting and define the patients in whom CCE is most suitable.

Full table
To the best of our knowledge, there has been only one prospective head-to-head study comparing CTC and CCE in patients with incomplete colonoscopy (38). In this study, 100 patients with previous incomplete colonoscopy underwent both CCE and CTC; conventional colonoscopy was eventually performed if one of the two techniques identified significant findings (mass lesion or at least one polyp ≥6 mm). CCE was able to achieve complete colonic evaluation in the vast majority of patients (98%), identifying significant polyps in a quarter of them. Compared to CTC, CCE identified more polyps with size thresholds of 6 and 10 mm [colon capsule relative sensitivity: 2.0, 95% confidence interval (CI): 1.34–2.98 and 1.67, 95% CI: 0.69–4.00 respectively]. No adverse events related to CCE or CTC were reported in this study. Interestingly, the study confirms the limitations of CTC in identifying flat/sessile lesions; all the 12 cases of discrepancies between CTC and CCE were non-polypoid lesions (2 of them ≥10 mm). Based on these findings, the authors concluded that both procedures are very effective in completing previous incomplete conventional colonoscopy, however, CCE seems to have a higher diagnostic yield. Nevertheless, since patients with negative CCE and CTC did not undergo repeat conventional colonoscopy, the false negative rate has not been assessed. Furthermore, despite employing a novel bowel preparation regimen, the rate of CCE examinations with adequate bowel preparation was only 83%. When CCE failed to visualize the entire colon (in 7% of patients in the study), it is not always possible to determine the length of colon examined based on results of previous conventional colonoscopy. Of note, even in the study from Spada et al. (38), a second conventional colonoscopy attempt, performed in patients with positive CCE or CTC, was always successful.
Taking into account the aforementioned limitations, as well as colon transit time (which ranges in the majority of studies between 75 and 200 min) (39-41), CCE does not show major advantages over conventional colonoscopy or CTC and may therefore not increase uptake for CRC screening, as hoped. At present conventional colonoscopy remains the reference standard for polyp detection, whereas CCE-2, similarly to CTC, may represent a valid alternative in average-risk patients refusing to undergo conventional colonoscopy or in those in which conventional colonoscopy did not allow a complete colon exploration (37). In a cost-benefit analysis by Health Quality Ontario, the cost-effectiveness of CCE compared to CTC is $26,750 per life-year, assuming an increased sensitivity of CCE. They estimated that the replacement of CTC with CCE would have moderate costs to the health care system (42).
Use of CCE for polyp detection
CTC has been proven to be more effective than barium enema (43,44) and as effective as conventional colonoscopy in the detection of colonic masses and large (i.e., ≥1 cm) polyps (45-47). Therefore, the US Preventive Services Task Force recently included CTC as a viable screening strategy for average risk asymptomatic 50–75 years subjects (48). Moreover, it has been also recommended by Scientific Societies as the imaging modality of choice in case of incomplete colonoscopy (49,50). Nevertheless, CTC requires X-ray exposure, and its accuracy in detecting colonic polyps significantly decreases when dealing with polyps smaller than 10 mm (46,47) or flat lesions (i.e., right sided sessile serrated lesions) (51,52). Furthermore, it can result in unnecessary diagnostic testing or treatment of incidental extra-colonic findings, which are identified in about 40% to 70% of CTC screening examinations (48).
A recently published comprehensive meta-analysis (27) pooled data from 14 studies: 7 of them (involving 1,128 patients) tested the accuracy of CCE-1 (first-generation of colon capsule), while the remaining 7 series (1,292 patients) tested that of CCE-2. This analysis has confirmed that the sensitivity values achieved by CCE-2, (i.e., 86% and 87% for ≥6 and ≥10 mm polyps, respectively) represent a clinically relevant improvement, compared to the corresponding values shown by CCE-1, (i.e., 58% and 54% for ≥6 and ≥10 mm polyps, respectively) (Table 2). In addition, the accuracy of CCE-2 reported in this paper favourably compares to that of CTC.

Full table
Non-CRC indications for CCE
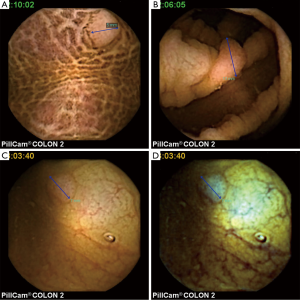
The ESGE has proposed potential future applications for CCE, although there is currently scarce data on these further indications. Areas where CCE could be applied include colon examination where optical conventional colonoscopy (OC) is contraindicated or refused, as well as the diagnosis and evaluation of IBD (37). In a prospective study (53) of 40 patients with Crohn’s disease (CD) undergoing both conventional colonoscopy and CCE, there was good agreement between the Crohn’s Disease Endoscopic Index of Severity (CDEIS) score using both modalities. However, CCE appeared to underestimate disease severity and there was poorer agreement in the distal colon with low specificity for colonic ulceration. Furthermore, the potential of the capsule to be a pantenteric examination tool could make it useful in assessing the entire gastrointestinal (GI) tract in CD patients. In a small study, the capsule was able to achieve complete GI tract visualization in 10/12 CD patients (54). There have been more studies on the use of CCE in ulcerative colitis (UC), showing good sensitivity and specificity for colonic inflammation. Studies detailing the use of CCE in IBD are summarized in Table 3.
Full table
Limitations faced by CCE
The aforementioned meta-analysis by Spada et al. (27) highlights some of the limitations of CCE in polyp detection compared to conventional colonoscopy. In the studies where CCE-2 was used, the completion rate was 90.5%. On the other hand, even after using preparation regimens with large volumes of polyethyleneglycol (PEG) solution and boosters, the rate of patients with adequate colon preparation remained around 80%. Unfortunately, both these parameters do not meet the thresholds established for conventional colonoscopy in quality improvement programs (66). In addition, although newly-developed polyp sizing software is now included in the RAPID® reading platform, it has never been validated. Therefore, polyp sizing using such software has been given a wider margin of error in recently published studies, potentially affecting the reliability of accuracy parameters calculation (39): the margin of error allowed for measurements made using CCE was 50% over or less than measurements made by conventional colonoscopy. Furthermore, the lack of insufflation and the underwater capsule navigation can affect the endoscopic appearance of certain lesions, e.g., flat lesions, making recognition and sizing difficult. Overall, the performance of CCE does not stack up favourably compared to increasingly sophisticated conventional colonoscopy techniques, which offer high definition imaging and are able to provide therapeutic intervention and biopsies.
Another important limitation of CCE is the need for bowel preparation to ensure adequate visualisation. Under current guidelines, a total of 4 L of PEG must be ingested prior to and during CCE (37). Furthermore, the use of sodium phosphate (NaP) as a booster precludes the use of CCE in patients at risk of NaP toxicity. Although a split-dosage regimen is advocated in order to improve the tolerability and efficacy of bowel preparation, it remains a highly unpleasant part of examination, negating the benefits of its noninvasive nature. Other booster preparations have been investigated but there is currently no conclusive evidence on their use (67).
Notably, CCE-2 reading is a time-consuming task, requiring intense and focused attention (68). This time and labour-intensiveness can significantly impact on everyday clinical activities. For instance, due to its patient acceptability, CCE-2 has been proposed as a possible “filter” or screening test for the selection of patients for conventional colonoscopy (39,41). Nevertheless, the amount of resources and manpower required for the provision of such service may place further strain on already-overstretched healthcare services. At present, there are no guidelines or formal training requirements for granting credentials to CCE readers. A recent meta-analysis from our group (unpublished data; under review) showed that properly trained physician extenders and/or specialist nurses could replace physicians in the reading of small-bowel capsule endoscopy videos. There is in fact no reason to suggest that the same should apply in CCE.
Future capsule colonoscopy hardware
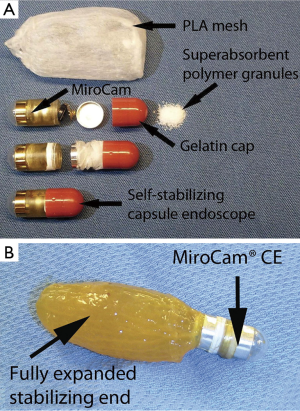
Although the PillCam colon remains the only commercial model for capsule colonoscopy, several experimental attempts have been made by other companies. Filip et al. (69) have presented a self-stabilizing capsule endoscope and tested it in a live canine model (Figure 5). The proposed modified capsule delivered a significant improvement in detection rates of colon suture markings when compared with an unmodified conventional capsule endoscope (MiroCam, IntroMedic, Seoul, Korea). However, since this presentation there has been no further information on further phase I studies and no market release date for this product has been officially announced.
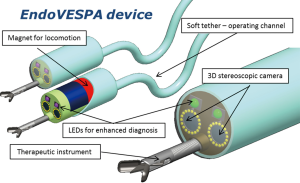
More recently, a magnetically-controlled capsule endoscopy (MCCE) system has been developed (70). A pilot study to assess its maneuverability and safety among volunteers undergoing CRC screening is under way. Another project funded by the European Union, the Endoscopic Versatile robotic guidancE, diagnoSis and theraPy of magnetic-driven soft-tethered endoluminAl robots (EndoVESPA) project, is in the process of developing a capsule-based colonoscopy device which is inserted similarly to a conventional colonoscope via the anus and moved through the colon to the caecum by external magnets. It retains a soft tether which allows air insufflation and instrumentation where necessary (Figure 6). A study assessing the feasibility of a magnetic robotically-driven capsule endoscopy has been detailed by Arezzo et al. (71).
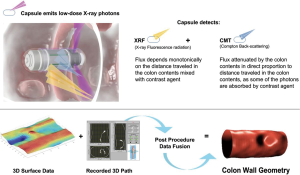
The recently developed Check-Cap® (Check-cap; Mount Carmel, Israel) is a capsule device that images the colon using low-dose radiation (total dose equivalent to a plain abdominal radiograph) and does not require bowel preparation (14,72,73) (Figure 7). The patient swallows the capsule with a small amount of a radio-opaque contrast agent, and can continue their everyday activities while data are transmitted to an external hand-held receiver for storage. The clinical performance of Check-Cap is under investigation and the device is still not commercially available.
Software developments
Current capsule endoscopy reading aids take the form of software which (I) improves the visibility of lesions; (II) selects frames for review in order to speed up reading times. Recent software developments are moving towards computer-aided diagnostic systems, aiming to increase diagnostic yield, reduce inter-observer variability and ultimately make the process of capsule endoscopy reading more efficient (74). Some of these have been trialled in other forms of endoscopy, including upper GI tract endoscopy for Barrett’s oesophagus (75), and small-bowel capsule endoscopy (76-79). A small number of studies have been published detailing the feasibility of automated polyp detection software in CCE (80,81), but much further development is required.
Conclusions
In 2009, we commented that conventional colonoscopy remains the cornerstone of a successful bowel screening programme either as a primary investigation or following a stool sample positive for faecal occult blood, not only as a diagnostic but also as a therapeutic tool (82). Of note, the bowel preparation regimen requires much adjustment to improve both the accuracy and patient acceptability of CCE. The addition of high definition imaging and further software image enhancement should allow further improvement of CCE validity. CCE has not as yet reached its full potential, and continuous further leaps are necessary to bring this technology to the point of offering accurate and sustainable panenteroscopy. The show must go on!
Acknowledgements
We would like to express our gratitude to Prof. Samuel N. Adler and Dr. Gastone Ciuti for providing Figure 1B and Figure 6, respectively.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Spada C, Riccioni ME, Petruzziello L, et al. The new PillCam Colon capsule: difficult colonoscopy? No longer a problem? Gastrointest Endosc 2008;68:807-8. [Crossref] [PubMed]
- Spada C, Hassan C, Costamagna G. Colon Capsule Endoscopy. Gastrointest Endosc Clin N Am 2015;25:387-401. [Crossref] [PubMed]
- Spada C, Barbaro F, Andrisani G, et al. Colon capsule endoscopy : What we know and what we would like to know. World J Gastroenterol 2014;20:16948-55. [Crossref] [PubMed]
- Weller D, Coleman D, Robertson R, et al. The UK colorectal cancer screening pilot: results of the second round of screening in England. Br J Cancer 2007;97:1601-5. [Crossref] [PubMed]
- Lee TJ, Rutter MD, Blanks RG, et al. Colonoscopy quality measures: experience from the NHS Bowel Cancer Screening Programme. Gut 2012;61:1050-7. [Crossref] [PubMed]
- Gavin DR, Valori RM, Anderson JT, et al. The national colonoscopy audit: a nationwide assessment of the quality and safety of colonoscopy in the UK. Gut 2013;62:242-9. [Crossref] [PubMed]
- Rees CJ, Gibson ST, Rutter MD, et al. UK key performance indicators and quality assurance standards for colonoscopy. Gut 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Lieberman DA, Weiss DG, Bond JH, et al. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med 2000;343:162-8. [Crossref] [PubMed]
- Regula J, Rupinski M, Kraszewska E, et al. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med 2006;355:1863-72. [Crossref] [PubMed]
- Bretthauer M, Kaminski MF, Løberg M, et al. Population-Based Colonoscopy Screening for Colorectal Cancer. JAMA Intern Med 2016;176:894. [Crossref] [PubMed]
- Shah HA, Paszat LF, Saskin R, et al. Factors Associated With Incomplete Colonoscopy: A Population-Based Study. Gastroenterology 2007;132:2297-303. [Crossref] [PubMed]
- Dafnis G, Granath F, Påhlman L, et al. Patient factors influencing the completion rate in colonoscopy. Dig Liver Dis 2005;37:113-8. [Crossref] [PubMed]
- Rizek R, Paszat LF, Stukel TA, et al. Rates of Complete Colonic Evaluation After Incomplete Colonoscopy and Their Associated Factors- A Population-Based Study. Med Care 2009;47:48-52. [Crossref] [PubMed]
- Villa NA, Pannala R, Pasha SF, et al. Alternatives to Incomplete Colonoscopy. Curr Gastroenterol Rep 2015;17:43. [Crossref] [PubMed]
- Imperiale TF, Wagner DR, Lin CY, et al. Risk of Advanced Proximal Neoplasms in Adults According To the Distal Colorectal Findings. N Engl J Med 2000;343:169-74. [Crossref] [PubMed]
- Brenner H, Chang-Claude J, Jansen L, et al. Role of colonoscopy and polyp characteristics in colorectal cancer after colonoscopic polyp detection: A population-based case-control study. Ann Intern Med 2012;157:225-32. [Crossref] [PubMed]
- Ridolfi TJ, Valente MA, Church JM. Achieving a complete colonic evaluation in patients with incomplete colonoscopy is worth the effort. Dis Colon Rectum 2014;57:383-7. [Crossref] [PubMed]
- Stoffel EM, Erichsen R, Froslev T, et al. Clinical and Molecular Characteristics of Post-Colonoscopy Colorectal Cancer: A Population-based study. Gastroenterol 2016. [Epub ahead of print].
- le Clercq CM, Winkens B, Bakker CM. Metachronous colorectal cancers result from missed lesions and non-compliance with surveillance. Gastrointest Endosc 2015;82:325-333.e2. [Crossref] [PubMed]
- Witte TN, Enns R. The difficult colonoscopy. Can J Gastroenterol 2007;21:487-90. [Crossref] [PubMed]
- Saunders BP, Fukumoto M, Halligan S, et al. Why is colonoscopy more difficult in women? Gastrointest Endosc 1996;43:124-6. [Crossref] [PubMed]
- Anderson JC, Gonzalez JD, Messina CR, et al. Factors that predict incomplete colonoscopy: Thinner is not always better. Am J Gastroenterol 2000;95:2784-7. [Crossref] [PubMed]
- Waye JD. Completing colonoscopy. Am J Gastroenterol 2000;95:2681-2. [Crossref] [PubMed]
- Hanson ME, Pickhardt PJ, Kim DH, et al. Anatomic factors predictive of incomplete colonoscopy based on findings at CT colonography. AJR Am J Roentgenol 2007;189:774-9. [Crossref] [PubMed]
- Bick BL, Vemulapalli KC, Rex DK. Regional center for complex colonoscopy: Yield of neoplasia in patients with prior incomplete colonoscopy. Gastrointest Endosc 2016;83:1239-44. [Crossref] [PubMed]
- Rex DK, Chen SC, Overhiser AJ, et al. Colonoscopy Technique in Consecutive Patients Referred for Prior Incomplete Colonoscopy. Clin Gastroenterol Hepatol 2007;5:879-83. [Crossref] [PubMed]
- Spada C, Pasha SF, Gross SA, et al. Accuracy of First- and Second-Generation Colon Capsules in Endoscopic Detection of Colorectal Polyps: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2016;14:1533-1543.e8. [Crossref] [PubMed]
- Fernández-Urién I, Ostiz M, Jiménez J. Avoiding incomplete conventional colonoscopies: PillCamTM COLON capsule endoscopy. Rev Esp Enferm Dig 2011;103:389-91. [Crossref] [PubMed]
- Triantafyllou K, Tsibouris P, Kalantzis C, et al. PillCam Colon capsule endoscopy does not always complement incomplete colonoscopy. Gastrointest Endosc 2009;69:572-6. [Crossref] [PubMed]
- Pioche M, De Leusse A, Filoche B, et al. Prospective multicenter evaluation of colon capsule examination indicated by colonoscopy failure or anesthesia contraindication. Endoscopy 2012;44:911-6. [Crossref] [PubMed]
- Nogales O, Lujan M, Nicolas D, et al. Utility of colon capsule endoscopy after an incomplete colonoscopy. Multicentric Spanish study. United European Gastroenterol J 2013;1:A344.
- Alarcón-Fernández O, Ramos L, Adrián-de-Ganzo Z, et al. Effects of Colon Capsule Endoscopy on Medical Decision Making in Patients With Incomplete Colonoscopies. Clin Gastroenterol Hepatol 2013;11:534-40.e1. [Crossref] [PubMed]
- Triantafyllou K, Viazis N, Tsibouris P, et al. Colon capsule endoscopy is feasible to perform after incomplete colonoscopy and guides further workup in clinical practice. Gastrointest Endosc 2014;79:307-16. [Crossref] [PubMed]
- Baltes P, Bota M, Albert JG, et al. Tu1557 PillCam Colon2® After Incomplete Colonoscopy- a Prospective Multi-Center Study. Gastrointest Endosc 2014;79:AB584. [Crossref]
- Spada C, Hassan C, Facg GC. Colon Capsule Endoscopy in Colorectal Cancer Screening: A Rude Awakening From a Beautiful Dream? Clin Gastroenterol Hepatol 2015;13:2302-4. [Crossref] [PubMed]
- Triantafyllou K, Beintaris I, Dimitriadis GD. Is there a role for colon capsule endoscopy beyond colorectal cancer screening ? A literature review. World J Gastroenterol 2014;20:13006-14. [Crossref] [PubMed]
- Spada C, Hassan C, Galmiche JP, et al. Colon capsule endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2012;44:527-36. [Crossref] [PubMed]
- Spada C, Hassan C, Barbaro B, et al. Colon capsule versus CT colonography in patients with incomplete colonoscopy: a prospective, comparative trial. Gut 2015;64:272-81. [Crossref] [PubMed]
- Rex DK, Adler SN, Aisenberg J, et al. Accuracy of capsule colonoscopy in detecting colorectal polyps in a screening population. Gastroenterology 2015;148:948-957.e2. [Crossref] [PubMed]
- Rondonotti E, Pennazio M. Colorectal polyp diagnosis: results with the second-generation colon capsule (CCE-2). Colorectal Dis 2015;17 Suppl 1:31-5. [Crossref] [PubMed]
- Rondonotti E, Borghi C, Mandelli G, et al. Accuracy of capsule colonoscopy and computed tomographic colonography in individuals with positive results from the fecal occult blood test. Clin Gastroenterol Hepatol 2014;12:1303-10. [Crossref] [PubMed]
- Palimaka S, Blackhouse G, Goeree R. Colon Capsule Endoscopy for the Detection of Colorectal Polyps: An Economic Analysis. Ont Health Technol Assess Ser 2015;15:1-43. [PubMed]
- Halligan S, Wooldrage K, Dadswell E, et al. Computed tomographic colonography versus barium enema for diagnosis of colorectal cancer or large polyps in symptomatic patients (SIGGAR): A multicentre randomised trial. Lancet 2013;381:1185-93. [Crossref] [PubMed]
- Sosna J, Sella T, Sy O, et al. Critical analysis of the performance of double-contrast barium enema for detecting colorectal polyps ≥ 6 mm in the era of CT colonography. AJR Am J Roentgenol 2008;190:374-85. [Crossref] [PubMed]
- Pickhardt PJ, Hassan C, Halligan S, et al. Colorectal Cancer: CT Colonography and Colonoscopy for Detection- Systematic Review and Meta-Analysis. Radiology 2011;259:393-405. [Crossref] [PubMed]
- Halligan S, Altman DG, Taylor SA, et al. CT colonography in the detection of colorectal polyps and cancer: systematic review, meta-analysis, and proposed minimum data set for study level reporting. Radiology 2005;237:893-904. [Crossref] [PubMed]
- Rosman AS, Korsten MA. Meta-analysis Comparing CT Colonography, Air Contrast Barium Enema, and Colonoscopy. Am J Med 2007;120:203-210.e4. [Crossref] [PubMed]
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for Colorectal Cancer. JAMA 2016;315:2564. [Crossref] [PubMed]
- AGA Clinical Practice and Economics Committee. Position of the American Gastroenterological Association (AGA) Institute on computed tomographic colonography. Gastroenterology 2006;131:1627-8. [Crossref] [PubMed]
- Spada C, Stoker J, Alarcon O, et al. Clinical indications for computed tomographic colonography: European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastrointestinal and Abdominal Radiology (ESGAR) Guideline. Endoscopy 2014;46:897-915. [Crossref] [PubMed]
- IJspeert JE, Tutein Nolthenius CJ, Kuipers EJ, et al. CT-Colonography vs. Colonoscopy for Detection of High-Risk Sessile Serrated Polyps. Am J Gastroenterol 2016;111:516-22. [Crossref] [PubMed]
- Togashi K, Utano K, Kijima S, et al. Laterally spreading tumors: Limitations of computed tomography colonography. World J Gastroenterol 2014;20:17552-7. [Crossref] [PubMed]
- D’Haens G, Löwenberg M, Samaan MA, et al. Safety and Feasibility of Using the Second-Generation Pillcam Colon Capsule to Assess Active Colonic Crohn’s Disease. Clin Gastroenterol Hepatol 2015;13:1480-6.e3. [Crossref] [PubMed]
- Boal Carvalho P, Rosa B, Dias de Castro F, et al. PillCam COLON 2© in Crohn’s disease: A new concept of pan-enteric mucosal healing assessment. World J Gastroenterol 2015;21:7233-41. [PubMed]
- Sung J, Ho K, Chiu H, et al. The use of Pillcam Colon in assessing mucosal inflammation in ulcerative colitis: a multicenter study. Endoscopy 2012;44:754-8. [Crossref] [PubMed]
- Hosoe N, Matsuoka K, Naganuma M, et al. Applicability of second-generation colon capsule endoscope to ulcerative colitis: A clinical feasibility study. J Gastroenterol Hepatol 2013;28:1174-9. [Crossref] [PubMed]
- Kobayashi T, Hosoe N, Matsuoka K, et al. Feasibility of the second-generation colon capsule endoscopy in patients with ulcerative colitis with a reduced preparation regimen. J Crohns Colitis 2013;7:S105. [Crossref]
- Manes G, Ardizzone S, Cassinotti A. PillCam Colon and ulcerative colitis: what do physicians need to know? Endoscopy 2013;45:325. [Crossref] [PubMed]
- Meister T, Heinzow HS, Domagk D, et al. Colon capsule endoscopy versus standard colonoscopy in assessing disease activity of ulcerative colitis: a prospective trial. Tech Coloproctol 2013;17:641-6. [Crossref] [PubMed]
- Shavrov AA, Kharitonova AIu. Second-generation colon capsule in small bowel and colon disorders in pediatrics. Vestn Ross Akad Med Nauk 2014.86-90. [Crossref] [PubMed]
- Singeap AM, Trifan A, Cojocariu C, et al. Colonoscopy and colon capsule endoscopy in inflammatory large bowel diseases: concordant or discordant results, alternative or complementary methods? J Crohns Colitis 2013;7:S117-8. [Crossref]
- Ye CA, Gao YJ, Ge ZZ, et al. PillCam colon capsule endoscopy versus conventional colonoscopy for the detection of severity and extent of ulcerative colitis. J Dig Dis 2013;14:117-24. [Crossref] [PubMed]
- Negreanu L, Smarandache G, Mateescu RB. Role of capsule endoscopy Pillcam COLON 2 in patients with known or suspected Crohn’s disease who refused colonoscopy or underwent incomplete colonoscopic exam: a case series. Tech Coloproctol 2014;18:277-83. [Crossref] [PubMed]
- Oliva S, Di Nardo G, Hassan C, et al. Second-generation colon capsule endoscopy vs. colonoscopy in pediatric ulcerative colitis: a pilot study. Endoscopy 2014;46:485-92. [Crossref] [PubMed]
- San Juan-Acosta M, Caunedo-Álvarez A, Argüelles-Arias F, et al. Colon capsule endoscopy is a safe and useful tool to assess disease parameters in patients with ulcerative colitis. Eur J Gastroenterol Hepatol 2014;26:894-901. [Crossref] [PubMed]
- Rembacken B, Hassan C, Riemann J, et al. Quality in screening colonoscopy: position statement of the European Society of Gastrointestinal Endoscopy (ESGE). Endoscopy 2012;44:957-68. [Crossref] [PubMed]
- Togashi K, Fujita T, Utano K, et al. Gastrografin as an alternative booster to sodium phosphate in colon capsule endoscopy : safety and efficacy pilot study. Endosc Int Open 2015;3:E659-61. [Crossref] [PubMed]
- Lo SK. How Should We Do Capsule Reading? Tech Gastrointest Endosc 2006;8:146-8. [Crossref]
- Filip D, Yadid-Pecht O, Muench G, et al. Suture marker lesion detection in the colon by self-stabilizing and unmodified capsule endoscopes: Pilot study in acute canine models. Gastrointest Endosc 2013;77:272-9. [Crossref] [PubMed]
- Gu H, Zheng H, Cui X, et al. Maneuverability and safety of magnetic-controlled capsule endoscopy system to examine the human colon under real-time monitoring by colonoscopy: a pilot study. Gastrointest Endosc 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Arezzo A, Menciassi A, Valdastri P, et al. Experimental assessment of a novel robotically-driven endoscopic capsule compared to traditional colonoscopy. Dig Liver Dis 2013;45:657-62. [Crossref] [PubMed]
- Chatrath H, Rex DK. Potential screening benefit of a colorectal imaging capsule that does not require bowel preparation. J Clin Gastroenterol 2014;48:52-4. [Crossref] [PubMed]
- Gluck N, Shpak B, Brun R, et al. A novel prepless X-ray imaging capsule for colon cancer screening. Gut 2016;65:371-3. [Crossref] [PubMed]
- Iakovidis DK, Koulaouzidis A. Software for enhanced video capsule endoscopy: challenges for essential progress. Nat Rev Gastroenterol Hepatol 2015;12:172-86. [Crossref] [PubMed]
- van der Sommen F, Zinger S, Curvers WL, et al. Computer-aided detection of early neoplastic lesions in Barrett’s esophagus. Endoscopy 2016;48:617-24. [Crossref] [PubMed]
- Li B, Meng MQ. Automatic polyp detection for wireless capsule endoscopy images. Expert Syst Appl 2012;39:10952-8. [Crossref]
- Bashar MK, Kitasaka T, Suenaga Y, et al. Automatic detection of informative frames from wireless capsule endoscopy images. Med Image Anal 2010;14:449-70. [Crossref] [PubMed]
- Bernal J, Sánchez FJ, Vilariño F. Towards automatic polyp detection with a polyp appearance model. Pattern Recognit 2012;45:3166-82. [Crossref]
- Ibrahim M, Van Gossum A. Novel imaging enhancements in capsule endoscopy. Gastroenterol Res Prac 2013;304723.
- Mamonov AV, Figueiredo IN, Figueiredo PN, et al. Automated Polyp Detection in Colon Capsule Endoscopy. IEEE Trans Med Imaging 2014;33:1488-502. [Crossref] [PubMed]
- Figueiredo PN, Figueiredo IN, Prasath S, et al. Automatic polyp detection in pillcam colon 2 capsule images and videos: preliminary feasibility report. Diagn Ther Endosc 2011;2011:182435.
- Koulaouzidis A, Plevris JN. Colon capsule endoscopy for detection of polyps and cancers: a step closer to non-invasive colon screening? J R Coll Physicians Edinb 2011;41:124-5. [Crossref] [PubMed]