Supra-therapeutic plasma concentrations of haloperidol induce moderate inhibition of lipopolysaccharide-induced interleukin-8 release in human monocytes
Introduction
Antipsychotics are used to treat psychotic symptoms and mood instability in a wide variety of neuropsychiatric disorders, including organic mental disorders such as dementia; intoxication or withdrawal symptoms due to ingestion of psychoactive substances; schizophrenia, schizotypal, delusional and schizoaffective disorders; bipolar disorders; tic disorders, in particular Tourette’s syndrome; and, in severe cases, depression and anxiety, obsessive-compulsive disorders, personality and eating disorders, and emotional instability associated with mental retardation (1,2). Anticonvulsants such as carbamazepine or valproic acid have traditionally been used to treat partial and generalized seizures (3), but are also used to successfully treat mood disorders (4). In addition, antipsychotics and anticonvulsants are frequently prescribed in combination (5). While the clinical efficacy and treatment-associated adverse events of both antipsychotics and anticonvulsants have been well investigated, knowledge about the effects of these psychopharmaceuticals on blood cell functions remains scarce and has been confined, primarily, to reports of rare cases of agranulocytosis or aplastic anaemia associated with clozapine or carbamazepine treatment (6,7). The lack of interest in the effects of antipsychotics and other psychopharmaceuticals on blood and immune cell functions is surprising, considering that a role for infection and immunity in the pathogenesis of schizophrenia has been proposed for decades. Torrey and Peterson formulated a hypothesis in 1973 that early infections with slow and latent human viruses may contribute to the development of schizophrenia in adult life (8). More recently, Karlsson and colleagues detected retroviral RNA in both cerebrospinal fluid and plasma of individuals with recent-onset schizophrenia (9,10). Maternal exposure to influenza viruses has been proposed as a risk factor for the development of schizophrenia (11) and for the onset of bipolar disorders with psychotic symptoms (12) in adult offspring.
Because many infections induce the synthesis and release of inflammatory mediators, it seems obvious to propose a role for such mediators in the development of schizophrenia and possibly other psychiatric disorders. Some inflammatory mediators, such as the pro-inflammatory cytokines interleukin-1β (IL-1β), interleukin-6 (IL-6) and tumour necrosis factor-α (TNF-α), which are normally associated with the peripheral immune system, are also synthesized and released by cells of the central nervous system where they assume a pivotal role in central nervous system development and function (13). A role for IL-1β, IL-6 and TNF-α in the pathogenesis of various psychiatric conditions, including schizophrenia, has been postulated based on the observation that these pro-inflammatory cytokines are pivotal in the development of sickness behaviour (14) and because the range of symptoms in sickness behaviour shows a significant overlap with symptoms of depression (14,15), which accompany many, if not all, psychiatric conditions (2).
This study examined the release of the pro-inflammatory chemokine interleukin-8 (IL-8) in Gram-negative lipopolysaccharide (LPS)-stimulated human whole blood ex vivo, in the presence or absence of the mood-stabilizing drugs carbamazepine and valproic acid and in the presence or absence of the antipsychotic drugs olanzapine, risperidone and haloperidol. At plasma concentrations exceeding the therapeutic range by three orders of magnitude, haloperidol reduced LPS-induced IL-8 production only moderately, while at equimolar concentrations, carbamazepine, valproic acid, olanzapine or risperidone remained inert with respect to LPS-stimulated IL-8 release. These findings suggest that the pharmaceuticals tested in this study possess clinically desirable inertness with respect to LPS-induced inflammatory processes.
Methods
Pharmaceuticals and reagents
Olanzapine (Zyprexa®, C17H20N4S, molecular weight, Mr 312.4 g/mol) was supplied in glass vials containing 10 mg of dried substance (Eli Lilly, Vernier, Switzerland). In accordance with the manufacturer’s recommendations, olanzapine was reconstituted in supplied H2O, and stock solutions (5 mg/mL) were used immediately. Risperidone (Risperdal®, C23H27FN4O2, Mr 410.5) and haloperidol (Haldol®, C21H23ClFNO2, Mr 375.9) were supplied in liquid form at concentrations of 1 mg/mL for risperidone and 2 mg/mL for haloperidol (Janssen-Cilag, Baar, Switzerland). Carbamazepine (Tegretol®, C15H12N2O, Mr 236.3) was supplied in liquid form at a concentration of 20 mg/mL (Novartis, Berne, Switzerland), and valproic acid (Depakine®, C8H16O2, Mr 144.2, pKa 4.8) in liquid form at a concentration of 60 mg/mL (Sanofi-Aventis, Meyrin, Switzerland). The following products were obtained from Sigma (St. Louis, MO, USA): Roswell Park Memorial Institute (RPMI) 1640 cell culture medium, containing L-glutamine and sodium bicarbonate; cell culture grade phosphate-buffered saline (PBS), supplied as a 10× concentrate; cell culture grade and γ-irradiated Escherichia coli 0111:B4 LPS, purified by gel-filtration chromatography; cell culture tested non-ionic detergent Tween-20; molecular biology grade H2O; sodium dodecyl sulfate (SDS), supplied as a 10% stock solution; hydrogen peroxide (H2O2), supplied as a 30% stock solution; and ABTS tablets, containing the diammonium salt of 2,2’-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid.
LPS stock solutions (1 mg/mL PBS) were stored at −20°C until further use. Heat-inactivated foetal bovine serum (FBS), low in haemoglobin and low in endotoxin, was supplied by Thermo Fisher Scientific (Waltham, MA, USA). IL-8 OptEIA™ enzyme-linked immunosorbent assay (ELISA) sets were obtained from Becton Dickinson (Franklin Lakes, NJ, USA) and contained unconjugated monoclonal anti-human IL-8 antibody (capture), biotinylated anti-human IL-8 monoclonal antibody (detection), streptavidin-horseradish peroxidase (HRP) conjugate (detection) and lyophilized baculovirus-expressed recombinant human IL-8 standard.
Materials
The median cubital vein was punctured to collect venous blood samples by using a BD Vacutainer® Safety-LokTM 21-gauge needle blood collection set with 8-inch tubing containing a 0.3-mL priming volume (Becton Dickinson). For LPS activation, venous blood was collected in 6-mL evacuated glass vials (Vacutainers) with yellow-coloured conventional stoppers. The vials contained 13.2 g/L trisodium citrate, 4.8 g/L citric acid and 14.7 g/L dextrose [acid citrate dextrose (ACD)] as an anticoagulant (Becton Dickinson). Monocyte counts in venous blood were determined by using 3-mL Vacutainers with lavender-coloured conventional stoppers; these vials contained the dipotassium salt of ethylenediaminetetraacetic acid (EDTA) as an anticoagulant (Becton Dickinson). Corning vacuum filter systems, containing cellulose acetate membranes, 0.2 µM in pore size, with a bottle volume of 1,000 mL, were obtained from Sigma. Parafilm® M, consisting of polyolefins and paraffin wax, was obtained from Brand (Wertheim, Germany). Polystyrene flat-bottom 96-well MaxiSorp™ microplates with high-capacity binding for immunoglobulin and containing maximum well volumes of 400 µL were from Invitrogen (Basel, Switzerland). Non-sterile standard microplate lids with cut-off corners (a Nunc product) were obtained through Thermo Fisher Scientific (Waltham, MA, USA).
Healthy donors
Volunteers were excluded if they were feeling unwell, if they reported cold symptoms or fever, or if they were under treatment for any condition at the time of blood sampling, including receiving any prescription drugs or over-the-counter drugs. Particular care was taken to exclude volunteers with any immune disorder, acute or chronic infection such as hepatitis B or C, coagulopathy, past thromboembolic complications, past thrombophlebitis, recent surgical procedures, past or current substance use, or a past or current psychiatric disorder. Also excluded were individuals who presented with sclerotic cubital veins. A healthy volunteer was defined as an individual who met none of the above-mentioned exclusion criteria.
Blood sampling
The collection of human whole blood involves the puncture of veins in the cubital fossa. Although a minimally invasive procedure, venipunctures are associated with health risks. Adherence to good clinical practice guidelines is recommended, as well as adherence to the ethical guidelines issued by the local ethics committee and to the statutory provisions that regulate health care services. Venipunctures may be performed by physicians or by licensed nurses, depending on the statutory provisions that regulate the sampling of venous blood from peripheral veins. It is advisable to have an anesthetist or emergency care physician on call during the entire procedure.
The person who performs the venipuncture should be sufficiently protected against infection with a hepatitis B virus (HBV). The Twinrix vaccine (GlaxoSmithKline) is recommended. Finally, in the event of a needle prick injury, immediate medical attention in an emergency department should be sought to evaluate the need for post-exposure prophylaxis after suspected exposure to HBV or human immunodeficiency virus, or post-exposure follow-up after suspected exposure to hepatitis C viruses. The person who performs the venipuncture should be familiar with the potential hazards and complications of venous access procedures, e.g., pain, bleeding, local infections, syncope and injuries from falls. Blood samples should not be collected in the laboratory, but rather, a consulting room is recommended that is properly equipped with a couch, a biohazard container for the disposal of needles and other blood-tainted material, suitable surface and skin disinfectants, kidney trays, tourniquets, alcohol swabs and adhesive bandages. Vacutainers should be labelled before blood is being drawn and coat, gloves and goggles should be worn for protection against blood-borne pathogens when handling human blood. Although in clinical practice, venipunctures are often performed in a sitting position, a supine position on a couch is recommended.
The procedures outlined above were adhered to as nine venous blood samples were obtained from eight healthy donors and collected in ACD and EDTA Vacutainers. Experiments were scheduled on three different days with four volunteers participating on day 1, three volunteers participating on day 22 and two volunteers participating on day 183, with one volunteer participating twice, on day 1 and on day 183. Blood from EDTA Vacutainers was used to determine the number of peripheral blood monocytes (normal range 160−950 per µL undiluted whole blood). As described previously (16), cells were stained for endogenous peroxidase and samples analyzed on an ADVIA 120 flow cytometer (Bayer Diagnostics, Munich, Germany).
LPS activation
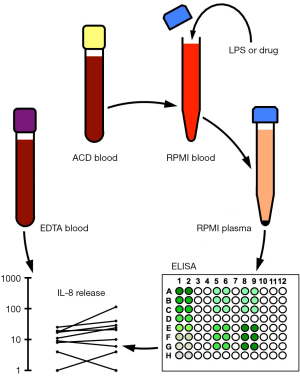
Aliquots of LPS stock solutions (1 mg/mL) were thawed and 5 µL added to 5 mL of PBS to yield the LPS working dilution (1 µg/mL). After whole blood was collected, a stopwatch was used to monitor time as long as samples contained live cells. ACD Vacutainers were opened in a negative pressure laminar flow unit by slowly twisting and pulling off the yellow stoppers. Blood from each donor was transferred to a 50-mL polypropylene tube by using a 5-mL disposable plastic pipette, which allowed volume measurement of transferred blood with an accuracy of 100 µL. Three volumes of RPMI 1640 medium, pre-warmed to 37 °C, were added and gently mixed by once pipetting up and down. RPMI-diluted whole blood was split into two 50-mL polypropylene tubes, one receiving 10 µL LPS working dilution per 1 mL diluted whole blood to yield a final LPS concentration of 10 ng/mL LPS, the other receiving 10 µL saline. Samples were split again into 15-mL polypropylene tubes, one tube receiving the psychotropic drug of interest at a final concentration of 100 µM, the other receiving an equal volume of saline. The resulting four samples from each donor, containing either saline alone (sample 1), psychotropic drug in saline (sample 2), LPS in saline (sample 3) or LPS plus psychotropic drug in saline (sample 4), were then incubated for 3 h at 37 °C in a 5% CO2 atmosphere. Samples were subsequently placed on ice and centrifuged at 400g for 5 min. Supernatants (diluted citrated plasma) were stored frozen at −20 °C or −70 °C until further analysis.
ELISA
The protocol used in this study is an adaption of the BD OptEIA assay for the detection of low analyte concentrations and requires the following working solutions. Coating buffer: 7.1 g NaHCO3 and 1.6 g Na2CO3 were added to 800 mL of deionized water (dH2O) and the solution adjusted to pH 9.5 by using 10 N NaOH. To obtain a 0.1 M sodium carbonate buffered solution, the volume was adjusted to 1,000 mL by using dH2O. Assay diluent: 10 mL FBS was added to 90 mL PBS. Wash buffer: 500 µL of Tween-20 was added to 1,000 mL of PBS. ABTS buffer: 51.5 mL of 0.2 M Na2HPO4 (28.4 g/L) was added to 48.5 mL of 0.1 M citric acid monohydrate (21 g/L) and the solution adjusted to 400 mL with dH2O to obtain a 0.05 M phosphate-citrate buffered solution, pH 5. ABTS substrate solution: one ABTS tablet, containing 10 mg ABTS, was dissolved in 100 mL ABTS buffer and aliquots stored at −20 °C. Stop solution: 1% SDS. Coating buffer, assay diluent and wash buffer were passed through 0.22-µM filters and the solutions were refrigerated (4 °C) and used within 1 week. Lyophilized IL-8 standard was brought to room temperature and 1,000 µL dH2O added. The resulting lot-specific concentration was calculated and aliquots stored at −70 °C.
The ELISA procedure was started by transferring 10-mL coating buffer to a 15-mL polypropylene tube. A lot-specific amount of IL-8 capture antibody was added and the mixture briefly vortexed and poured in a plastic reservoir (e.g., polypropylene lids of pipette tip boxes). An eight-channel micropipette was used to transfer 100 µL of coating buffer to each well of a 96-well MaxiSorp plate. Particular care was taken to avoid touching the bottom of the plate. The MaxiSorp plate was covered with a microplate lid, sealed with Parafilm M and kept at 4 °C overnight. The plate was then brought to room temperature and the capture antibody solution discarded by inverting and flicking the plate over a sink. The top of the plate was blotted dry by briefly touching the inverted plate on absorbent paper. Wells were washed three times with 300 µL of wash buffer before 100 µL of assay diluent was added to each well (blocking) and left for 1 h at room temperature. Wells were washed again three times with 300 µL of wash buffer and the last wash left in the wells. An aliquot of the IL-8 stock solution was thawed out and diluted in assay diluent to obtain a concentration of 1,000 pg/mL in a final volume of 500 µL. After being vortexed, 250 µL of the resulting dilution was transferred to 250 µL of assay diluent. This procedure was continued to obtain a serial dilution down to 16 pg/mL. Sample aliquots that had been stored frozen in 1.5-mL Eppendorf tubes were thawed, vortexed and centrifuged for 5 min in a tabletop Eppendorf 5415D centrifuge at maximum speed, i.e., 13,200 rpm. Note that samples may contain transmissible blood-borne pathogens and should be handled as biohazardous material. The final wash solution was discarded and 100 µL of samples and standards were added in duplicate by using a single-tip micropipette. This step is critical, as working at a fast pace is required to avoid drying of wells. The plate was covered with a lid, sealed with Parafilm M and left for 2 h at room temperature. Samples and standards were then discarded by inverting the plate on absorbent paper and the soaked paper discarded in a biohazard container. Wells were washed five times with 300 µL of wash buffer and the last wash left in the wells. Note that wash buffer contains the detergent Tween-20, which inactivates transmissible blood-borne agents. A lot-specific amount of biotinylated IL-8 detection antibody was added to 10 mL of assay diluent and the mixture briefly vortexed. A lot-specific amount of streptavidin-HRP conjugate was then added and the mixture vortexed again. The wash solution was discarded and 100 µL of the detection-HRP mix transferred to each well. The plate was covered with a lid, sealed with Parafilm M and left for 1 h at room temperature. Wells were washed seven times with 300 µL of wash buffer and the last wash left in the wells. Immediately before use, 10 µL of H2O2 stock solution was added to 10 mL of ABTS substrate solution. After discarding the wash solution, 100 µL of the substrate solution was added to each well.
Optical density (OD) was read at 405 nm (17,18) with a reference setting at 550 nm, using a Titertek Multiskan device (ICN Pharmaceuticals, Costa Mesa, CA, USA). The ABTS substrate is ideal for kinetic (multiple) measurements, as colour develops slowly. Most optical readers convert relative OD values into concentrations (pg/mL). Alternatively, sample concentrations can be calculated manually by using OD means of standard dilutions and subtracting the background mean (OD mean of wells containing 0 pg IL-8). Resulting standard OD values (x-axis) are plotted against the corresponding standard concentrations (y-axis) on double logarithmic paper and a best-fit linear regression line is drawn. Note that linearity may be lost at high standard concentrations. Sample concentrations can then be estimated with an accuracy that is limited by the logarithmic paper. For better accuracy, sample concentrations may be calculated by placing the mean OD value (x-axis) into the linear regression formula y = ax + b, where b=0 (background subtraction) and a =1 for a regression line angle of 45°. If sample analyte concentrations fall outside the linear range, the samples should be diluted and reanalyzed. Alternatively, sample concentrations can be approximated by linear interpolation by using standard values above and below the OD value of interest. Comparing the standard analytical procedures mentioned above, linear interpolation yielded the most accurate results. Therefore, in this study, OD values were converted to concentrations (pg/mL) by using linear interpolation formulas.
Finally, monocyte counts (number per milliliter undiluted EDTA blood) obtained from routine haematology analysis were used to convert IL-8 concentrations (pg/mL) into IL-8 amounts (pg) released by 105 monocytes. Note that blood from two individuals may contain significantly different numbers of monocytes, yet may produce similar IL-8 plasma concentrations upon LPS stimulation. Normalizing IL-8 amounts to monocyte numbers defines the activity of LPS-stimulated monocytes.
Statistical analysis
Multiple variables were analyzed by using a one-way analysis of variance (ANOVA) for correlated samples and post-ANOVA pairwise comparisons were performed by using the Tukey honestly significant difference (HSD) procedure. Two-tailed probabilities of less than 0.05 (P<0.05) were considered statistically significant.
Results
Previously, we have examined various chemical structures for their ability to either increase or decrease LPS-induced monocyte responses in RPMI-diluted whole blood (19). The purpose of the present study was to examine the effects of five frequently used psychopharmaceuticals on LPS-induced IL-8 release in RPMI-diluted peripheral venous human whole blood. LPS-activated monocytes release many pro-inflammatory mediators that can be quantified by using microplate-based ELISA protocols similar to that described in this study. The advantages of using IL-8 as a marker for LPS-induced pro-inflammatory processes in human whole blood have been discussed previously (16). TNF-α, for instance, is a very sensitive and early marker for LPS activation and has been used to demonstrate heparin-mediated enhancement of the LPS response in cultured human monocytes (20). In human whole blood, however, the release of TNF-α in the presence of 10 ng/mL LPS exceeds amounts of 1 ng per 105 monocytes after an incubation period of 4 h at 37 °C (data not shown). This near-maximum release in the presence of LPS limits the use of TNF-α as a suitable marker for the identification of drugs that modify LPS responses. Heparin, for instance, enhances LPS responses by a factor of one-half or less when TNF-α is measured (data not shown), but by more than five-fold when IL-8 release is quantified (21). Other limitations apply to IL-1β, which appears slowly after LPS stimulation, requiring prolonged incubation times, or to IL-6, which is produced at relatively low levels after LPS activation, thereby hampering the detection of moderate LPS-inhibiting effects (22).
To mimic in vivo conditions as closely as possible, whole blood was diluted ex vivo with RPMI cell culture medium to decrease the viscosity of the blood for further handling, yet without subjecting monocytes to physical (23,24) or immunological enrichment (25,26) or to cytokine-induced differentiation procedures, which require prolonged incubation periods (26), rendering the results more difficult to interpret. The experimental design is illustrated in Figure 1. One volume of citrated peripheral venous whole blood from healthy volunteers was diluted with three volumes of RPMI cell culture medium and incubated for 3 h at 37 °C in the presence or absence of 10 ng/mL LPS with or without the addition of 100 µM of the mood-stabilizing drugs carbamazepine and valproic acid (Figure 2) or the antipsychotics olanzapine, risperidone and haloperidol (Figure 3). In the presence of saline alone, incubation of diluted whole blood ex vivo resulted in baseline IL-8 releases ranging from 11±9 pg/105 monocytes (Figure 3B) to 14±10 pg/105 monocytes (Figure 2A). In the presence of carbamazepine, valproic acid, olanzapine, risperidone or haloperidol, IL-8 secretion remained close to baseline levels, ranging from 12±7 pg/105 monocytes (Figure 3A) to 28±35 pg/105 monocytes (Figure 3C). The presence of risperidone or haloperidol yielded somewhat higher IL-8 amounts, with values of 25±20 pg/105 monocytes (Figure 3B) and 28±35 pg/105 monocytes (Figure 3C, inset Figure 1), respectively. However, none of the five psychopharmaceuticals increased or decreased the amounts of IL-8 in RPMI-diluted plasma to levels reaching statistical significance when compared with the baseline condition in the presence of saline alone. Adding LPS (10 ng/mL) resulted in a substantial IL-8 increase, ranging from 760±357 pg/105 monocytes (Figure 3A) to 890±444 pg/105 monocytes (Figure 2A). When the mood-stabilizing anticonvulsants carbamazepine and valproic acid were added along with LPS, the amounts of IL-8 were similar to those obtained with LPS alone. Indeed, analysis by the ANOVA Tukey HSD procedure revealed that the differences were statistically non-significant (Figure 2). Adding the antipsychotic drugs olanzapine or risperidone to samples containing LPS resulted in IL-8 levels that were, again, statistically indistinguishable from IL-8 levels obtained by samples containing LPS alone (Figure 3A,B). However, the addition of haloperidol resulted in a statistically significant, albeit moderate, inhibition of LPS-induced IL-8 secretion (Figure 3C). Haloperidol, at the supra-therapeutic concentration of 100 µM, combined with 10 ng/mL LPS, resulted in the release of 436±293 pg IL-8/105 monocytes, whereas the addition of LPS alone produced 875±399 pg IL-8/105 monocytes (Figure 3C). Therefore, haloperidol inhibited LPS-stimulated IL-8 secretion in RPMI-diluted whole blood by approximately 52% (Figure 3, inset).
These results confirm the early findings by Moots and colleagues on haloperidol’s inhibiting effect on LPS-mediated pro-inflammatory responses in human monocytes (27). However, in this study, haloperidol-mediated inhibition was moderate and required supra-therapeutic drug concentrations; moreover, it was observed only for haloperidol and not for carbamazepine, valproic acid, olanzapine or risperidone. Taken together, the results presented here argue in favour of these five psychopharmaceuticals being inert, rather than pro-inflammatory, and thus bearing a clinically desirable property.


Discussion
The results of this study show that haloperidol exhibits moderate inhibition of LPS-induced IL-8 release in human whole blood monocytes and further demonstrate that carbamazepine, valproic acid, olanzapine and risperidone lack such modifying effects on LPS-induced monocytic IL-8 release. We previously showed that the appearance of IL-8 in LPS-stimulated human whole blood primarily reflects the activation of monocytes but not neutrophils (16), thus defining IL-8 as a suitable marker for determining LPS-enhancing or LPS-neutralizing effects of a chemical or drug of interest.
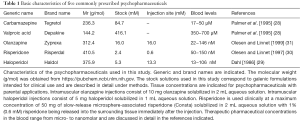
If pharmaceutical drugs are to be compared for their ability to interfere with cellular signalling pathways, then equimolar drug concentrations must be used. In this study, equimolar drug concentrations of 100 µM were used, first, because the therapeutic blood levels for carbamazepine and valproic acid are within the micromolar range (28) and second, because the concentrations of olanzapine, risperidone and haloperidol, applied intramuscularly, are within the millimolar range with micromolar concentrations occurring in the surrounding tissue. In the peripheral bloodstream, however, the concentrations of olanzapine, risperidone and haloperidol remain within a nanomolar range (29-31) (Table 1). The finding that 100 µM haloperidol reduced LPS-induced IL-8 release in monocytes by half after an incubation period of 3 h suggests that, in vivo, this reduction remains confined to tissue where drug concentrations of this magnitude occur, i.e., in the adjacent tissue after intramuscular haloperidol injections. Rare injection site reactions have been reported after intramuscular haloperidol administration (32); however, injection site abscesses after intramuscular injection of haloperidol decanoate appear to be rarer (Haldol® Injection, Janssen, package inset). The results presented here are consistent with previous observations that severe injection site complications occur infrequently after intramuscular olanzapine (33) or risperidone (34) injections.
Full table
Whereas this study reports a moderately inhibiting effect of haloperidol on immune responses evoked by Gram-negative LPS, such inhibition, when evoked by other mechanisms, may be beneficial for the host. Moots and colleagues described the case of a female patient with both rheumatoid arthritis and mania, the latter being treated with haloperidol (27). Unexpectedly, markers of systemic inflammation decreased during haloperidol treatment and a clinical improvement of the active synovitis occurred as long as haloperidol was administered. This observation prompted the authors to investigate the effect of haloperidol on the release of the pro-inflammatory cytokines IL-1β and TNF-α in whole blood that had been obtained from one healthy donor and diluted with RPMI 1640 medium before stimulation with LPS. They found that the release of both cytokines in diluted whole blood was inhibited by haloperidol and, furthermore, that the release of IL-1β in cultured THP-1 cells showed similar inhibition when haloperidol was added. Unfortunately, these authors used heparin as an anticoagulant in their whole blood experiment, introducing an unwanted bias, as heparin strongly enhances LPS-induced pro-inflammatory responses in human whole blood (21).
Studies investigating pro-inflammatory cytokines in patients with schizophrenia, with or without drug treatment, have yielded conflicting results. For example, Maes and colleagues examined venous plasma samples from 14 schizophrenia patients, 10 mania patients and 21 healthy control subjects and found that the pro-inflammatory cytokine IL-6 was elevated in schizophrenia patients but not in mania patients or in healthy controls. Moreover, these authors found that treatment of schizophrenia patients with the antipsychotics haloperidol, perphenazine or thioridazine reduced IL-6 levels, whereas in mania patients treated with valproic acid, no change in IL-6 levels occurred (35). However, investigating a sample of 10 patients receiving haloperidol monotherapy, Pollmächer and colleagues found no change in the plasma levels of either IL-6 or TNF-α during the course of treatment (36). Applying an experimental approach with minimal sample processing, de Witte and colleagues determined the cytokine profile in peripheral venous blood by using serum tubes that had been kept clotting for 2 h at room temperature before analysis. The study compared healthy volunteers with antipsychotic-naïve individuals who were having their first episode of schizophrenia and found no differences between the groups in the levels of TNF-α (37).
The work by Zhang and colleagues demonstrates that initial positive results from non-randomized studies can be lost when study conditions are chosen to be more robust. In their pilot study, Zhang and colleagues collected venous whole blood from 70 drug-free schizophrenia patients and 30 healthy control subjects and determined the serum levels of IL-6 and IL-8. The authors found that the proportion of schizophrenia patients exhibiting detectable levels of IL-6 or IL-8 was larger than that of control subjects with measurable amounts of IL-6 or IL-8. Furthermore, blood concentrations of both inflammatory mediators were significantly higher in schizophrenia patients than in healthy controls (38). Subsequently, Zhang and colleagues conducted a 12-week randomized controlled trial with an intention-to-treat sample of 78 schizophrenia patients. After a 2-week lead-in period with placebo treatment, the authors randomized the patients into two groups, providing treatment with either risperidone or haloperidol. Measuring serum concentrations of IL-6 and IL-8, the authors found no pre- to post-treatment differences in the levels of either cytokine in either group (39).
Meta-analyses of larger numbers of studies confirmed these conflicting results. Miller and colleagues published a meta-analysis of 40 studies on the association of blood cytokine levels and schizophrenia (40). The authors reported elevated levels of IL-1β, IL-6 and TNF-α in first-episode psychosis and elevated levels of IL-6, IL-8 and TNF-α in acutely relapsed schizophrenia patients. Notably, treatment with antipsychotics led to normalization of the levels of IL-1β, IL-6 and TNF-α in these patients. However, different results were reported by Tourjman and colleagues, who published a meta-analysis of 23 studies on the effect of antipsychotic treatment on blood cytokine levels in schizophrenia (41). While excluding studies involving in vitro leukocyte stimulation, the authors found that IL-1β and INF-γ, but not IL-6 and TNF-α, were reduced during treatment. The work by Zakharyan and Boyajyan seems to suggest that results on the association between cytokines and schizophrenia are contradictory, partly because of the different methodical procedures used to determine blood levels of cytokines (42).
Conclusions
In conclusion, this study provides evidence for beneficial inertness, rather than pro-inflammatory properties, of the frequently used psychopharmaceuticals carbamazepine, valproic acid, olanzapine and risperidone. The inhibition of LPS-induced IL-8 release by 100 µM haloperidol was moderate, suggesting that these effects on pro-inflammatory processes are confined to intramuscular injection sites. Since its original synthesis by Bert Hermans at Janssen Laboratories in 1958 (43), haloperidol, both orally and intramuscularly, has been administered to a great number of patients, suggesting that its effect on immune functions compromises neither safety nor tolerability. However, elucidating the mechanism by which haloperidol interferes with the LPS signaling pathway in a moderate fashion may lead to novel insights into the pharmacodynamics of other butyrophenones and define pharmaceutical targets in the LPS activation pathway that could be beneficial for the development of future sepsis drugs.
Acknowledgements
The study was funded in part by Healthcare Project Management (HPM), Geneva, Switzerland, as indicated under Footnote. Special thanks are due to the volunteers who participated in this study. The author thanks Barbara Every, ELS, of BioMedical Editor, for English language editing.
Footnote
Conflicts of Interest: The author wishes to disclose his involvement as an investigator in the ObsIM study, a prospective observational study of the safety and effectiveness of intramuscular psychotropic medication in patients who are acutely agitated (Code: F1D-EW-S018). The study was headed by HPM on behalf of Eli Lilly (Suisse) SA, Geneva, Switzerland. The author participated in the study from 2004 through 2005 while affiliated with the Zurich Psychiatric University Hospital, Switzerland, and received personal fees commensurate with the amount of acquired data. Fees were transferred to a funding account provided by the University of Zurich (account number 34080310 on behalf of Herbert Bosshart) and used exclusively for research purposes.
Ethical Statement: The study was approved by the local Ethics Committee and written informed consent was obtained from all healthy volunteers.
References
- The ICD-10 classification of Mental and Behavioural Disorders. World Health Organization. Geneva, 1992. Available online: www.who.int/classifications/icd/en/
- Sadock BJ, Sadock VA. Kaplan and Sadock’s Comprehensive Textbook of Psychiatry. 9th Edition. Philadelphia: Lippincott Williams & Wilkins, 2003.
- Mattson RH, Cramer JA, Collins JF. A comparison of valproate with carbamazepine for the treatment of complex partial seizures and secondarily generalized tonic-clonic seizures in adults. The Department of Veterans Affairs Epilepsy Cooperative Study No. 264 Group. N Engl J Med 1992;327:765-71. [Crossref] [PubMed]
- Belmaker RH. Bipolar disorder. N Engl J Med 2004;351:476-86. [Crossref] [PubMed]
- Stahl SM, Grady MM. A critical review of atypical antipsychotic utilization: comparing monotherapy with polypharmacy and augmentation. Curr Med Chem 2004;11:313-27. [Crossref] [PubMed]
- Pellock JM. Carbamazepine side effects in children and adults. Epilepsia 1987;28 Suppl 3:S64-70. [Crossref] [PubMed]
- Alvir JM, Lieberman JA, Safferman AZ, et al. Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med 1993;329:162-7. [Crossref] [PubMed]
- Torrey EF, Peterson MR. Slow and latent viruses in schizophrenia. Lancet 1973;2:22-4. [Crossref] [PubMed]
- Karlsson H, Bachmann S, Schröder J, et al. Retroviral RNA identified in the cerebrospinal fluids and brains of individuals with schizophrenia. Proc Natl Acad Sci U S A 2001;98:4634-9. [Crossref] [PubMed]
- Karlsson H, Schröder J, Bachmann S, et al. HERV-W-related RNA detected in plasma from individuals with recent-onset schizophrenia or schizoaffective disorder. Mol Psychiatry 2004;9:12-3. [Crossref] [PubMed]
- Kneeland RE, Fatemi SH. Viral infection, inflammation and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2013;42:35-48. [Crossref] [PubMed]
- Canetta SE, Bao Y, Co MD, et al. Serological documentation of maternal influenza exposure and bipolar disorder in adult offspring. Am J Psychiatry 2014;171:557-63. [Crossref] [PubMed]
- Deverman BE, Patterson PH. Cytokines and CNS development. Neuron 2009;64:61-78. [Crossref] [PubMed]
- Dantzer R. Cytokine, sickness behavior, and depression. Immunol Allergy Clin North Am 2009;29:247-64. [Crossref] [PubMed]
- Musselman DL, Miller AH, Porter MR, et al. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. Am J Psychiatry 2001;158:1252-7. [Crossref] [PubMed]
- Bosshart H, Heinzelmann M. Arginine-rich cationic polypeptides amplify lipopolysaccharide-induced monocyte activation. Infect Immun 2002;70:6904-10. [Crossref] [PubMed]
- Bruice TC, Zipplies MF, Lee WA. The pH dependence of the mechanism of reaction of hydrogen peroxide with a nonaggregating, non-mu-oxo dimer-forming iron (III) porphyrin in water. Proc Natl Acad Sci U S A 1986;83:4646-9. [Crossref] [PubMed]
- Re R, Pellegrini N, Proteggente A, et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999;26:1231-7. [Crossref] [PubMed]
- Bosshart H, Heinzelmann M. Targeting bacterial endotoxin: two sides of a coin. Ann N Y Acad Sci 2007;1096:1-17. [Crossref] [PubMed]
- Heinzelmann M, Platz A, Flodgaard H, et al. Endocytosis of heparin-binding protein (CAP37) is essential for the enhancement of lipopolysaccharide-induced TNF-alpha production in human monocytes. J Immunol 1999;162:4240-5. [PubMed]
- Heinzelmann M, Bosshart H. Heparin binds to lipopolysaccharide (LPS)-binding protein, facilitates the transfer of LPS to CD14, and enhances LPS-induced activation of peripheral blood monocytes. J Immunol 2005;174:2280-7. [Crossref] [PubMed]
- Schindler R, Mancilla J, Endres S, et al. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood 1990;75:40-7. [PubMed]
- Banfalvi G. Cell cycle synchronization of animal cells and nuclei by centrifugal elutriation. Nat Protoc 2008;3:663-73. [Crossref] [PubMed]
- Frikeche J, Simon T, Brissot E, et al. Impact of valproic acid on dendritic cells function. Immunobiology 2012;217:704-10. [Crossref] [PubMed]
- Kowalski J, Blada P, Kucia K, et al. Neuroleptics normalize increased release of interleukin- 1 beta and tumor necrosis factor-alpha from monocytes in schizophrenia. Schizophr Res 2001;50:169-75. [Crossref] [PubMed]
- Chen ML, Tsai TC, Wang LK, et al. Risperidone modulates the cytokine and chemokine release of dendritic cells and induces TNF-α-directed cell apoptosis in neutrophils. Int Immunopharmacol 2012;12:197-204. [Crossref] [PubMed]
- Moots RJ, Al-Saffar Z, Hutchinson D, et al. Old drug, new tricks: haloperidol inhibits secretion of proinflammatory cytokines. Ann Rheum Dis 1999;58:585-7. [Crossref] [PubMed]
- Palmer SM, Kaufman RA, Salamone SJ, et al. Cobas Integra: clinical laboratory instrument with continuous and random-access capabilities. Clin Chem 1995;41:1751-60. [PubMed]
- Dahl SG. Plasma level monitoring of antipsychotic drugs. Clinical utility. Clin Pharmacokinet 1986;11:36-61. [Crossref] [PubMed]
- Olesen OV, Linnet K. Simplified high-performance liquid chromatographic method for determination of risperidone and 9-hydroxyrisperidone in serum from patients comedicated with other psychotropic drugs. J Chromatogr B Biomed Sci Appl 1997;698:209-16. [Crossref] [PubMed]
- Olesen OV, Linnet K. Olanzapine serum concentrations in psychiatric patients given standard doses: the influence of comedication. Ther Drug Monit 1999;21:87-90. [Crossref] [PubMed]
- Hamann GL, Egan TM, Wells BG, et al. Injection site reactions after intramuscular administration of haloperidol decanoate 100 mg/mL. J Clin Psychiatry 1990;51:502-4. [PubMed]
- Atkins S, Detke HC, McDonnell DP, et al. A pooled analysis of injection site-related adverse events in patients with schizophrenia treated with olanzapine long-acting injection. BMC Psychiatry 2014;14:7. [Crossref] [PubMed]
- Lindenmayer JP, Jarboe K, Bossie CA, et al. Minimal injection site pain and high patient satisfaction during treatment with long-acting risperidone. Int Clin Psychopharmacol 2005;20:213-21. [Crossref] [PubMed]
- Maes M, Bosmans E, Calabrese J, et al. Interleukin-2 and interleukin-6 in schizophrenia and mania: effects of neuroleptics and mood stabilizers. J Psychiatr Res 1995;29:141-52. [Crossref] [PubMed]
- Pollmächer T, Hinze-Selch D, Fenzel T, et al. Plasma levels of cytokines and soluble cytokine receptors during treatment with haloperidol. Am J Psychiatry 1997;154:1763-5. [Crossref] [PubMed]
- de Witte L, Tomasik J, Schwarz E, et al. Cytokine alterations in first-episode schizophrenia patients before and after antipsychotic treatment. Schizophr Res 2014;154:23-9. [Crossref] [PubMed]
- Zhang XY, Zhou DF, Zhang PY, et al. Elevated interleukin-2, interleukin-6 and interleukin-8 serum levels in neuroleptic-free schizophrenia: association with psychopathology. Schizophr Res 2002;57:247-58. [Crossref] [PubMed]
- Zhang XY, Zhou DF, Cao LY, et al. Changes in serum interleukin-2, -6, and -8 levels before and during treatment with risperidone and haloperidol: relationship to outcome in schizophrenia. J Clin Psychiatry 2004;65:940-7. [Crossref] [PubMed]
- Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry 2011;70:663-71. [Crossref] [PubMed]
- Tourjman V, Kouassi É, Koué MÈ, et al. Antipsychotics' effects on blood levels of cytokines in schizophrenia: a meta-analysis. Schizophr Res 2013;151:43-7. [Crossref] [PubMed]
- Zakharyan R, Boyajyan A. Inflammatory cytokine network in schizophrenia. World J Biol Psychiatry 2014;15:174-87. [Crossref] [PubMed]
- Granger B. The discovery of haloperidol. Encephale 1999;25:59-66. [PubMed]


