Continuous monitoring of the liver graft temperature: relationship between bacterial contamination of the perfusion fluid and early outcome
Introduction
Opportunistic infections after solid organ transplantation represent one of the major causes of morbidity and mortality in liver transplant (LT) recipients (1). Many reports in the literature describe various modes of microorganism transmission (2-6).
A potential mechanism of infection would be an infected donor, contamination at the time of the infusion and/or packing, back-table procedure, and finally during the transplantation, all are potential sources of infection. Nevertheless, in most reports there is no mention of the role that the graft temperature may play in bacterial transmission. Moreover, a sterile preservation medium is prone to the colonization from microorganisms.
The aim of our study is to analyze the incidence and significance of infections in the preservation solution in relation with the graft temperature. The second aim is to analyze the impact of graft temperature on the clinical infections and the ischemia reperfusion injury.
Methods
Between September 2015 and December 2015, we prospectively studied all liver grafts transplanted at San Camillo Hospital Center in Rome. In all cases, donor data, retrieval data and post-operative data were collected. A post-reperfusion biopsy was routinely performed before abdominal wall closure.
Temperature monitoring of the liver graft was performed using infrared thermometer with fixed emissivity infrared (IR Etekcity®, LaserGrip 1080), without direct contact with the liver surface. The thermometer was placed 15 cm far from the surface of the liver, corresponding in all cases to the left hepatic lobe. The determination of the temperature was carried out in seven successive stages: two minutes after the cold perfusion start, at the end of the retrieval, before the graft packing, at the arrival time in the operative room, at the beginning and at the end of the back-table surgery, and before reperfusion.
Six fluid samples of the preservation fluid were taken under aseptic conditions during liver retrieval: at the beginning and at the end of the retrieval, at the arrival time in the operative room, at the beginning and at the end of the back-table surgery, and when the liver was pulled off the ice.
Fluid samples were processed at the microbiology laboratory of Department of Microbiology, of San Camillo-Forlanini Hospital.
In all cases we included aerobic and anaerobic microbiological analyses. All data were collected using special forms filled in by the surgeon at the end of the retrieval surgery and after the transplant at collected into a prospective database and analyzed.
Results
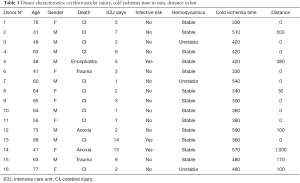
Sixteen donors were prospectively included in this study: 9 males and 7 females. Donor characteristics are resumed in Table 1. Mean donor age was 59.8 years (range, 31−84 years). In 11 cases donor death was caused by cerebrovascular accidents, two cases by anoxia, two cases by trauma and one case by infection (bacterial meningoencephalitis). Mean intensive care unit (ICU) stay was 4.7 days (range, 1−14 days). Only three donors had proven infectious risk. Cold ischemia time was 424 min (range, 300−590 min). Mean distance from donor to our Center was 142 km (range, 0−1,000 km).
Full table
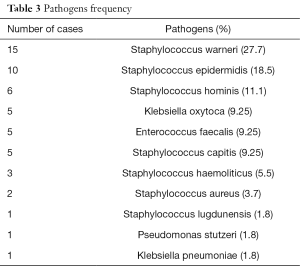
Infected preservation fluid samples were found in 36.25% of cases (29/80). The microorganisms found are resumed in Table 2. The most frequently isolated bacteria were Staphylococci (warneri, epidermidis, hominis) followed by Klebsiella Oxitoca (9.25%) and Enterococcus faecalis (9.5%). In only one case a Klebsiella pneumonia sensitive to carbapenems was isolated. The frequency of microorganisms is resumed in Table 3.

Full table
Full table
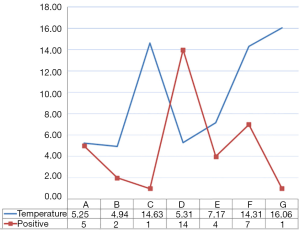
The liver graft temperature monitoring shows variations in four different phases: at the beginning of retrieval, before the graft packing, at the beginning and at the end of the backtable surgery (Figure 1). As shown in the graph in Figure 2, the temperature changes during the various stages of the study do not correspond to an increase in contamination of the preservation fluid.
In all cases a graft biopsy to evaluate ischemia reperfusion injury was performed before the abdominal wall closure. In 15 cases lymphocytic infiltration into portal areas have been recognized, not associated with necrosis, macrovascular steatosis <30%. In one case, interlobular necrosis with lymphocytic infiltration portal biliary was observed.
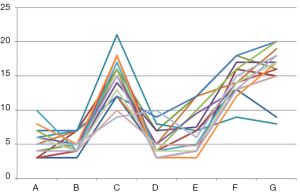
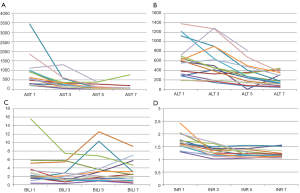
Post-operative blood tests were collected at day 1, 3, 5 and 7 posts LT and analyzed. There was no correlation between the functionality of the graft and the temperature of the perfusion fluid (Figure 3). In particular, no transplant patient developed primary graft non-function or delayed graft function. One patient died on the seventh postoperative day for irreversible brain edema due to fulminant hepatitis from mushrooms for which he had received the graft as a super-urgent transplant.
Discussion
Although many studies have compared the preservation solutions, using as variables acute rejection, graft dysfunction, and complications of the biliary tract, we used the graft temperature to observe the incidence of fluid perfusion contamination on LT and its consequences on early surgical outcomes for the recipient. Opportunistic infections after solid organ transplantation represent one of the most important causes of morbidity and mortality. In some series, the incidence of infections after LT was as high as 80% and was more common during the first 3 months, which coincides with the highest level of immunosuppressive therapy (7). The perfusion fluid used to preserve the liver graft represents a potential medium in which microorganisms can easily grow. In our study a temperature increase was observed between the end of the retrieval and the packing of the organ, and from the back-table until the reperfusion. On the other hand, a higher frequency of contamination of the fluid was found at the arrival to our Center. In spite of a low temperature, this contamination was observed after the graft transport, probably because this is the longest time in which the liver stays in the preservation fluid (Figure 2). We did not find any correlation between the fluid contamination and the distance between the donor hospital and our center; also the mean cold ischemia time was similar. Certainly, particular attention must be put into the graft management from the end of the perfusion to the graft packing in order to minimize graft manipulation and temperature increase.
Superficial saprophytic flora such as staphylococci and streptococci were mostly found in the perfusion samples (Table 3). Highly virulent pathogens (Staphylococcus aureus, Enterococcus Faecalis, Pseudomonas and Klebsiella) were found. Multi-drugs resistant species are linked to a high morbidity and mortality of transplanted patients (8,9). In our cases we had no multi-drugs resistant species.
In this study, a clinically evident infection directly related to the contamination of the preservation fluid by the same microorganism was never observed.
Conclusions
In conclusion, it is important to maintain a constant temperature during the procedure. Asepsis during retrieval surgery should be the primary goal. We did not find a correlation between graft temperature, microbiology results of the preservation solution and early post-transplant follow up.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by ethics committee of San Camillo-Forlanini (No. 90/14) and written informed consent was obtained from all patients.
References
- Ruiz P, Gastaca M, Gonzalez J, et al. Incidence and clinical relevance of bacterial contamination in preservation solution for liver transplantation. Transplant Proc 2009;41:2169-71. [Crossref] [PubMed]
- Cerutti E, Stratta C, Romagnoli R, et al. Bacterial- and fungal-positive cultures in organ donors: clinical impact in liver transplantation. Liver Transpl 2006;12:1253-9. [Crossref] [PubMed]
- Ranghino A, Diena D, Simonato F, et al. Clinical impact of bacterial contamination of perfusion fluid in kidney transplantation. Springerplus 2016;5:7. [Crossref] [PubMed]
- González-Segura C, Pascual M, García Huete L, et al. Donors with positive blood culture: could they transmit infections to the recipients? Transplant Proc 2005;37:3664-6. [Crossref] [PubMed]
- Janny S, Bert F, Dondero F, et al. Microbiological findings of culture-positive preservation fluid in liver transplantation. Transpl Infect Dis 2011;13:9-14. [Crossref] [PubMed]
- Grąt M, Ligocka J, Lewandowski Z, et al. Incidence, pattern and clinical relevance of microbial contamination of preservation fluid in liver transplantation. Ann Transplant 2012;17:20-8. [Crossref] [PubMed]
- del Pozo JL. Update and actual trends on bacterial infections following liver transplantation. World J Gastroenterol 2008;14:4977-83. [Crossref] [PubMed]
- Wakelin SJ, Casey J, Robertson A, et al. The incidence and importance of bacterial contaminants of cadaveric renal perfusion fluid. Transpl Int 2005;17:680-6. [Crossref] [PubMed]
- Sharma AK, Smith G, Smith D, et al. Clinical outcome of cadaveric renal allografts contaminated before transplantation. Transpl Int 2005;18:824-7. [Crossref] [PubMed]