A rare presentation of acute heart failure secondary to aggressive uterine leiomyosarcoma metastatic to the myocardium initially diagnosed as hypertrophic obstructive cardiomyopathy
Introduction
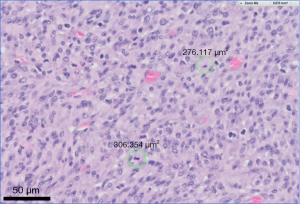
Uterine sarcoma accounts for 3–9% of all uterine malignant neoplasms and has a 2-fold higher incidence in black women as compared to white women (1,2). Cellular atypia and abundant mitoses (≥10 per 10 high power fields) as seen in this case (Figure 1) are associated with an increased risk for metastases (3). Metastases to the heart are infrequently reported with a handful of cases in the literature. We present a case of a 51-year-old woman with aggressively metastatic uterine leiomyosarcoma causing acute heart failure 4 months after initial presentation.
Case presentation
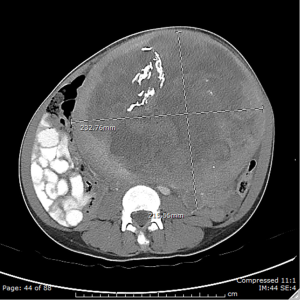
A 51-year-old woman visiting from Nigeria, presented to the gynecology clinic with progressive abdominal distension, chest discomfort, and dyspnea with a history of fibroids. A transthoracic echocardiogram (TTE) on June 4, 2015 showed grade 1 diastolic dysfunction (Figure 2). Ultrafast computed tomography (CT) of the chest/abdomen/pelvis showed a uterine mass and diffuse lung nodules (Figure 3). She underwent a thoracotomy and wedge biopsy on June 15, 2016, with pathology and immunohistochemistry consistent with metastatic leiomyosarcoma.
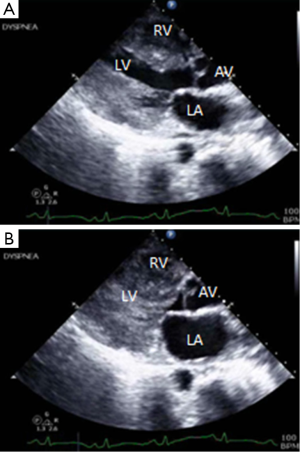
The patient then underwent a total abdominal hysterectomy with bilateral salpingo-oopherectomy (TAHBSO) consistent with high-grade leiomyosarcoma. She was started on gemcitabine and docetaxel with palliative radiation to the chest for diffuse metastases. She had multiple recurrent admissions due to chest pain despite palliative radiation therapy. A TTE on July 14, 2015 showed increased wall thickness with a pattern of severe left ventricular hypertrophy with dynamic obstruction in the mid cavity of the left ventricle managed with beta blockers. Repeat CT of the chest/abdomen/pelvis on July 19, 2015 was consistent with metastases to the muscles of the bilateral abdominal walls, para-spinal musculature, gluteal musculature, upper legs, thighs, and hamstrings.
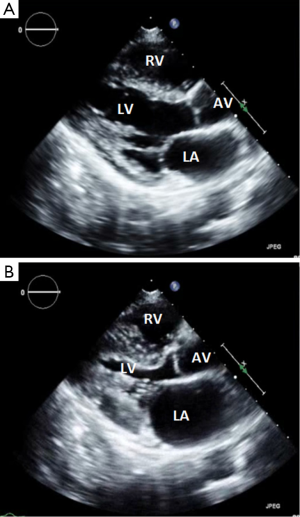
Because of complaints of worsening diffuse pain, she underwent a bone scan on July 21, 2015 which revealed osseous metastases to the pelvis, thoracolumbar spine, and bilateral patella. Her course was also complicated with gastrointestinal bleeding secondary to metastases to the stomach, duodenum, and colon, confirmed on biopsy. She was discharged and readmitted with dyspnea and fluid overload with acute decompensated heart failure along with headaches, with brain metastases confirmed on magnetic resonance imaging (MRI) on September 1, 2015. A TTE confirmed a 43 times 15 mm mobile mass in the right ventricle with severe left ventricular hypertrophy and abnormal myocardial specular pattern (Figure 4). The patient died within 10 days of presentation due to right ventricular failure, on September 9, 2015.
Discussion
Uterine sarcomas accounts for 3–9% of all malignant neoplasms of the uterus (1,2). Uterine sarcomas are rare with an incidence of 0.36 per 100,000 woman-years in the United States from 1979 to 2001 (4) or 3 to 7 per 100,000 US population from 1989 to 1999 (5), based on data from the Surveillance, Epidemiology and End Results National Cancer database. The mean age at diagnosis is approximately 60 years old with a 2-fold higher incidence of leiomyosarcomas in blacks as compared to their white counterparts (5).
Hereditary conditions associated with leiomyosarcoma include (HLRCC) syndrome, a rare autosomal dominant syndrome with associated renal cell carcinoma caused by mutations in fumarate hydratase, presenting with cutaneous and uterine leiomyomas and an aggressive form of papillary renal cell cancer. Premenopausal women with HLRCC syndrome are especially at high-risk of developing uterine sarcomas (6). Survivors of childhood retinoblastoma also have a higher risk of developing sarcomas (7).
Uterine sarcomas can have various initial presentations. In one of the largest series with data from a national registry in Norway, uterine sarcomas presented as postmenopausal bleeding (31% to 46%), premenopausal abnormal uterine bleeding (27% to 34%), abdominal pain (4% to 13%), abdominal distension (8% to 17%), urinary symptoms (1% to 2%), and is asymptomatic (1% to 2%) (1).
As compared to the more common endometrial carcinomas, uterine sarcomas, especially leiomyosarcomas, are more aggressive with a poorer prognosis, but many present at an earlier stage. In one series of over 1,000 cases, the stage distribution for uterine sarcoma was: stage I (60%), stages II and III (16%), and IV (22%) (1). They are referred to as homologous or heterologous. The majority are homologous, differentiating in ways similar to normal uterine tissues as compared to heterologous tumors, which contain elements with non-native differentiation (e.g., skeletal muscle, cartilage, bone).
In order to determine the metastatic potential of uterine smooth muscle tumors sarcoma, the Stanford criteria were created. These include cellular atypia, mitosis, and “coagulative necrosis”. In the largest retrospective study, which resulted in the development of these criteria, the presence of two of these three features indicated a risk of metastatic spread of >10% (3). Other common features, which are not diagnostic but may be used in borderline cases, include hypercellularity and an infiltrative border.
Metastatic uterine leiomyosarcoma with cardiac involvement is exceedingly rare with scattered reports in the literature (8-11). In one study of 131 patients, the most frequent metastatic sites were: lung (67.7%), cranial/intracranial (16.2%), skin/soft tissues (15.3%), and bone (13.8%) (12). The lack of available literature offers a challenge for early diagnosis and management of similar cases.
In order to assess cardiac tumors, various modalities are present including echocardiography, cardiac MRI, CT, and coronary angiography. Echocardiography can usually identify the presence of a mass, its mobility, and any obstruction to the circulation. Transesophageal echocardiography (TEE) is superior to TTE due to the proximity of the esophagus to the heart, the absence of obstructing structures including the lungs and bones, along with the ability to use high-frequency imaging transducers affording superior spatial resolution (13).
Cardiac MRI and CT are also used as noninvasive modalities to assess cardiac tumors. Although both offer high resolution imaging, cardiac MRI is superior as the T1 and T2 weighted sequences can help with determining the type of tumor by reflecting the chemical microenvironment within a tumor (14-16). In those with primary tumors with concern for metastatic spread, positron emission tomography has also been useful in identifying cardiac involvement (17-20), along with investigating lipomatous septal hypertrophy (21) or atrial myxoma (22).
More invasive modalities for assessing cardiac tumors include coronary angiography and transvenous biopsy. Coronary angiography is especially helpful in identifying the blood supply of tumors arising from the epicardial surfaces (14). As for transvenous biopsy, there are limited data to the associated risks and benefits in identifying cardiac tumors.
Previous cases of uterine leiomyosarcoma reported resolution of heart failure with surgical excision of the cardiac tumor (8-11). However, due to the late presentation in this case, and the aggressive metastatic disease, the patient was not recommended for surgical intervention. This case report highlights the importance of early detection and management in patients with similar presentations.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed consent: Informed consent cannot be obtained from anyone and is not indicated. There is no possible way that this patient could be identified by reading the case report or by looking at the figures.
References
- Nordal RR, Thoresen SO. Uterine sarcomas in Norway 1956-1992: incidence, survival and mortality. Eur J Cancer 1997;33:907-11. [Crossref] [PubMed]
- Tropé CG, Abeler VM, Kristensen GB. Diagnosis and treatment of sarcoma of the uterus. A review. Acta Oncol 2012;51:694-705. [Crossref] [PubMed]
- Bell SW, Kempson RL, Hendrickson MR. Problematic uterine smooth muscle neoplasms. A clinicopathologic study of 213 cases. Am J Surg Pathol 1994;18:535-58. [Crossref] [PubMed]
- Toro JR, Travis LB, Wu HJ, et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978-2001: An analysis of 26,758 cases. Int J Cancer 2006;119:2922-30. [Crossref] [PubMed]
- Brooks SE, Zhan M, Cote T, et al. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989-1999. Gynecol Oncol 2004;93:204-8. [Crossref] [PubMed]
- Launonen V, Vierimaa O, Kiuru M, et al. Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc Natl Acad Sci U S A 2001;98:3387-92. [Crossref] [PubMed]
- Yu CL, Tucker MA, Abramson DH, et al. Cause-specific mortality in long-term survivors of retinoblastoma. J Natl Cancer Inst 2009;101:581-91. [Crossref] [PubMed]
- Moreno Antón F, Casado Herraez A, Puente Vázquez J, et al. Cardiac metastasis from uterine leiomyosarcoma. Clin Transl Oncol 2006;8:375-8. [Crossref] [PubMed]
- Peng YJ, Hueng GG, Lee HS. Acute heart failure as manifestation of metastatic uterine leiomyosarcoma to the heart and lung. Heart Lung 2004;33:46-9. [Crossref] [PubMed]
- Calleja AM, Wellnitz CV, Alharthi MS, et al. Extensive cardiac metastases secondary to uterine leiomyosarcoma. J Am Soc Echocardiogr 2009;22:1419.e5-7. [Crossref] [PubMed]
- Nguyen SK, Wong F. Right atrial metastasis of uterine leiomyosarcoma causing obstructive shock. Curr Oncol 2012;19:e292-4. [Crossref] [PubMed]
- Bartosch C, Afonso M, Pires-Luís AS, et al. Distant Metastases in Uterine Leiomyosarcomas: The Wide Variety of Body Sites and Time Intervals to Metastatic Relapse. Int J Gynecol Pathol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Engberding R, Daniel WG, Erbel R, et al. Diagnosis of heart tumours by transoesophageal echocardiography: a multicentre study in 154 patients. European Cooperative Study Group. Eur Heart J 1993;14:1223-8. [Crossref] [PubMed]
- Vander Salm TJ. Unusual primary tumors of the heart. Semin Thorac Cardiovasc Surg 2000;12:89-100. [Crossref] [PubMed]
- Kaminaga T, Takeshita T, Kimura I. Role of magnetic resonance imaging for evaluation of tumors in the cardiac region. Eur Radiol 2003;13 Suppl 4:L1-10. [Crossref]
- Araoz PA, Eklund HE, Welch TJ, et al. CT and MR imaging of primary cardiac malignancies. Radiographics 1999;19:1421-34. [Crossref] [PubMed]
- García JR, Simo M, Huguet M, et al. Usefulness of 18-fluorodeoxyglucose positron emission tomography in the evaluation of tumor cardiac thrombus from renal cell carcinoma. Clin Transl Oncol 2006;8:124-8. [Crossref] [PubMed]
- Gates GF, Aronsky A, Ozgur H. Intracardiac extension of lung cancer demonstrated on PET scanning. Clin Nucl Med 2006;31:68-70. [Crossref] [PubMed]
- Kim JH, Jung JY, Park Y, et al. Non-small cell lung cancer initially presenting with intracardiac metastasis. Korean J Intern Med 2005;20:86-9. [Crossref] [PubMed]
- Buchmann I, Wandt H, Wahl A, et al. FDG PET for imaging pericardial manifestations of Hodgkin lymphoma. Clin Nucl Med 2003;28:760-1. [Crossref] [PubMed]
- Fan CM, Fischman AJ, Kwek BH, et al. Lipomatous hypertrophy of the interatrial septum: increased uptake on FDG PET. AJR Am J Roentgenol 2005;184:339-42. [Crossref] [PubMed]
- Agostini D, Babatasi G, Galateau F, et al. Detection of cardiac myxoma by F-18 FDG PET. Clin Nucl Med 1999;24:159-60. [Crossref] [PubMed]