Anatomy essentials for laparoscopic inguinal hernia repair
Laparoscopic inguinal hernia repair is performed more and more nowadays because of its mini-invasive nature and demonstrated good results. Laparoscopic procedures are especially suitable for recurrent and bilateral inguinal hernia (1,2). The major procedures include intraperitoneal onlay mesh (IPOM) repair, transabdominal preperitoneal (TAPP) repair and total extraperitoneal (TEP) repair. The anatomy of these procedures is totally different from traditional open procedures because they are performed from different direction. Laparoscopic operations for inguinal hernia are carried out intraperitoneally or in preperitoneal space. Surgeons must understand important anatomic acknowledge of the operation area under laparoscopic views before they begin to perform these procedures, otherwise it will be very risky to cause complications such as bleeding, nerve damage, insufficient repair and recurrence. The main anatomic points are discussed as followed.
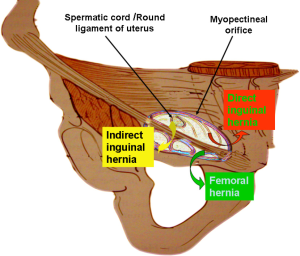
Myopectineal orifice
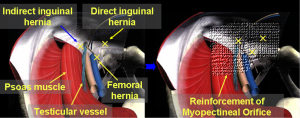
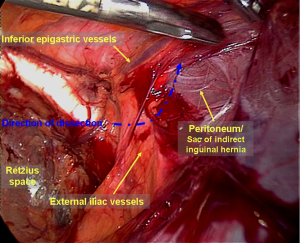
This anatomic region was originally coined by Dr. Fruchaud, a French researcher, in 1956. Direct inguinal hernias, oblique inguinal hernias and femoral hernias are all caused by weakness of the abdominal transverse fascia in myopectineal orifice (Figure 1). The inguinal ligament divides the myopectineal orifice into two regions: the suprainguinal region and the subinguinal region. The spermatic cord or the round ligament of the uterus runs through the suprainguinal region, while the femoral nerve, the femoral artery, the femoral vein and the femoral canal run through the subinguinal region. The deep layer of the myopectineal orifice is closed off by the abdominal transverse fascia, which surrounds the spermatic cord, and the femoral sheath, which passes through the myopectineal orifice. A single-side repair of the myopectineal orifice can simultaneously and completely repair the site of anatomical weakness for inguinal, direct and femoral hernias. This approach is also the principle of adult laparoscopic inguinal hernia repair (Figure 2).
Transverse fascia
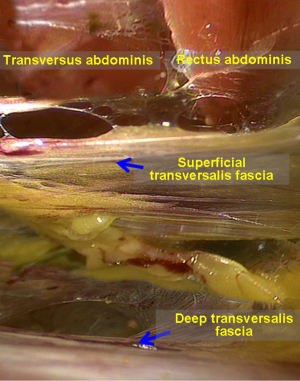
The transverse fascia is a complicated and contentious anatomical structure. Overall, it is a thin aponeurotic membrane that lies between the rectus abdominis, the deep layer of the transverse abdominal muscle, and the peritoneum. Some researchers have described it as a two-layer structure, while other researchers have described it as a single-layer structure; some researchers have reported that the transverse fascia is thick and dense in structure, while other researchers have reported that it is thin in structure. In fact, the structure of the transverse fascia is not important in the TAPP approach or the IPOM repair; therefore, the transverse fascia is often neglected. However, the transverse fascia has important clinical relevance in the TEP patch repair; a correct understanding of the structure of the transverse fascia can help ensure a smooth operation. According to our clinical observations, the transverse fascia can be divided into two layers in the majority of patients. To facilitate this description, the anatomical structures of the lower anterior abdominal wall (especially with respect to the transverse fascia) are defined as follows (Figure 3).
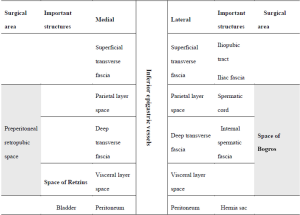
The transverse fascia in the lower anterior abdominal wall is divided into two layers (Figure 4). The superficial transverse fascia tightly covers the inner surface of the anterior abdominal muscles, but it is thin and has no clinical value in a hernia repair. The deep transverse fascia, beneath the superficial transverse fascia, covers the parietal peritoneum and is relatively thick and dense in 50% of patients (i.e., it is thin and loose in the other 50% of patients). The space between the superficial and deep transverse fascia is the parietal space. The superficial and deep transverse fasciae extend to the inguinal region and cover the blood vessels under the abdominal wall (both sides). Then, they blend with the anterior abdominal wall at the site lateral to the inferior epigastric blood vessels. The lateral transverse fascia continues to ascend to the posterior lower edge of the inguinal ligament and then blends with the iliac fascia. The medial transverse fascia is attached to the pubic bone, the pectineus muscle and Cooper’s ligament. The deep transverse fascia becomes a funnel-shaped structure that extends downward to cover the spermatic cord structures (the vas deferens, the testicular vessels and the hernia sac of the oblique inguinal) at the internal inguinal ring and becomes the internal spermatic fascia entering the inguinal canal. Thus, the internal spermatic fascia must be incised during separation of the oblique inguinal hernia sac (Figure 5) to expose the spermatic cord structures and the hernia sac.
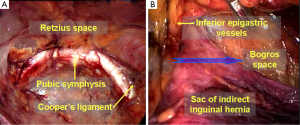
Preperitoneal retropubic space and extraperitoneal space posterior to the transverse fascia (space of Bogros)
These two spaces are potential non-natural cavities under the lower anterior abdominal wall, and they lie in between the superficial transverse fascia and the peritoneum (Figure 6). They are created by blunt separation when performing a laparoscopic inguinal hernia repair.
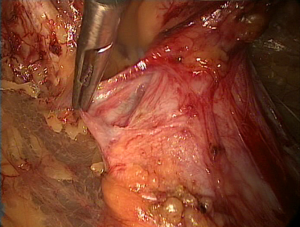
The preperitoneal retropubic space is located in the midline of the lower abdomen with the superficial transverse fascia and the pubic bone anteriorly, the bladder posteriorly, the umbilicus level superiorly, the pelvic floor muscles inferiorly, and the inferior epigastric arteries laterally. It is filled with loose connective tissue and fat, and there are no obvious blood vessels. The space is easily separated; the pubic symphysis and the shiny Cooper’s ligament are readily visible after slight blunt separation. Usually, the preperitoneal retropubic space is considered to be equivalent to the space of Retzius. However, the space of Retzius originally referred to the space formed by the fold of the tight fusion of the deep transverse fascia and the peritoneum between the bladder and the peritoneum, which includes the bladder and is filled with loose connective tissue. In fact, to obtain a more capacious preperitoneal retropubic space, the surgeon needs to incise the deep transverse fascia that is attached to the pubic bone and Cooper’s inguinal ligament and enter the visceral space. Therefore, the preperitoneal retropubic space should include the space of Retzius, a part of the visceral space and a part of the parietal space.
The space of Bogros is located lateral to the space of Retzius and is bound anteriorly by the superficial transverse fascia, medially by the inferior epigastric blood vessels, laterally by the pelvic wall, and posteriorly by the psoas muscle, the external iliac vessels and the femoral nerve. During laparoscopic inguinal hernia repair, the space of Bogros is explored to access the iliac fossa as well as to make it easier to open the lateral mesh and lay it flat. During surgery, after the preperitoneal retropubic space is separated, care should be taken that the deep transverse abdominal fascia is tightly attached to the anterior abdominal wall at the site lateral to the inferior epigastric blood vessels when separating the space of Bogros (Figure 7). Thus, the deep transverse fascia should be incised at the attachment site to enter the space of Bogros. The separation is required to access the space of Bogros due to the relatively tight fusion of the transverse abdominal fascia and the peritoneum.
Important anatomic structures and landmarks
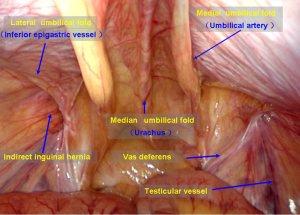
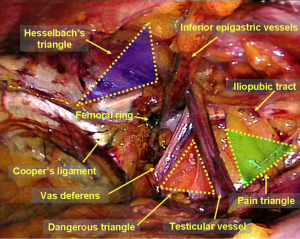
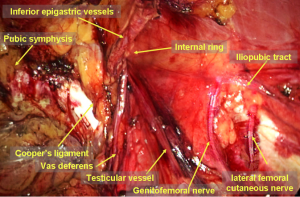
During laparoscopic inguinal hernia repair, it is important to recognize the following important structures in the abdominal cavity: the median umbilical fold, the medial umbilical fold, the lateral umbilical fold, Hesselbach’s triangle, the internal inguinal ring and the femoral ring. These structures are the landmarks for making a correct diagnosis and performing accurate surgeries (Figure 8). Other anatomical structures in the extraperitoneal space that must be recognized include the pubic symphysis, Cooper’s ligament, the corona mortis, the inferior epigastric vessels, the vas deferens/the round ligament of the uterus, the testicular vessels, the iliopubic tract, the dangerous triangle (triangle of doom) and the triangle of pain (Figure 9).
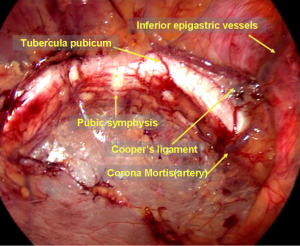
The pubic symphysis is the first exposed anatomical landmark at separation of the space of Retzius and is the medial reference line when placing mesh.
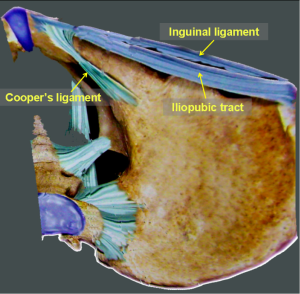
Cooper’s ligament (also known as the pectineal ligament) is easier to identify because it is white, shiny and tough tendinous tissue. It is an extension of the lacunar ligament, running infero-laterally along the pectineal line and attaching to the pectineal line. Cooper’s ligament is a structure that can hold a mesh and tacks.
One or a number of anastomotic vessels between the inferior epigastric or the external iliac vessels and the obturator arteries or veins, namely, the corona mortis, can be visualized at the site 5 cm away from the pubic symphysis, arching over Copper’s ligament (Figure 10). The corona mortis includes arteries and veins, most of which travel alone and leave the pelvic cavity via the obturator canal. During surgery, significant hemorrhage may occur, and hemostasis may be difficult to achieve if the corona mortis vessels are accidentally cut because they may retract into the obturator canal. Therefore, the corona mortis is known as the “crown of death” to remind surgeons to be alert during a procedure such as a separation and fixation on Copper’s ligament.
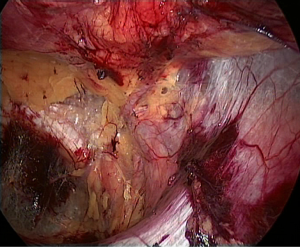
The separation continues laterally along Cooper’s ligament and the dark blue external iliac vein; the white, elastic, pulsating external iliac artery can be seen after passing the corona mortis. The slightly thin inferior epigastric arteries and veins can be seen at the top of the external iliac vessels. Most of the inferior epigastric arteries are branches of the external iliac arteries or veins. The inferior epigastric artery usually runs with two veins along the back of the rectus abdominis muscle toward the umbilicus. The identification of the inferior epigastric vessels is very important before accessing the space of Bogros. Separating between the inferior epigastric vessels and the deep transverse abdominal fascia is the only approach to correctly gain access to the space of Bogros (Figure 11). Otherwise, it is easy to accidentally damage the inferior epigastric vessels or pierce the peritoneum, which may cause difficulties while performing laparoscopic surgery or even require conversion to open surgery.
During a laparoscopic inguinal hernia repair, the dangerous triangle (the triangle of doom) refers to a triangular area bound by the vas deferens, the testicular vessels and the peritoneal fold. Within the boundaries of this area, you can find the external iliac artery and vein. Separation in this area is risky in the setting of an external iliac vascular malformation or aneurysm.
The triangle of pain is a triangular area located lateral to the dangerous triangle and bound by the iliopubic tract, the testicular vessels and the peritoneal fold. This area from lateral to medial includes the lateral femoral cutaneous nerve, the femoral branch of the genitofemoral nerve and the femoral nerve, which runs on the surface of the psoas muscle and the iliac muscle. Most of these nerves pass through the deep surface of the iliopubic tract to innervate the corresponding area of the perineum and thigh (Figure 12). The femoral nerve is 6 cm above the inguinal ligament and is not easily injured because it is covered by the psoas muscle. The lateral femoral cutaneous nerve runs just below the iliac fascia and enters the thigh in the 1- to 4-cm-wide region infero–medial to the anterior superior iliac spine under the iliopubic tract. During separation of the space of Bogros, avoiding piercing the iliac fascia and exposing the nerves is one of the most effective methods to reduce the incidence of postoperative chronic neuropathic pain. Clinical data have shown that the lateral femoral cutaneous nerve and the femoral branch of the genitofemoral nerve are more commonly damaged. Minor damage can result in abnormal sensation in the area innervated by these nerves. Such symptoms can resolve spontaneously in 2–4 weeks. However, these abovementioned nerves can suffer major damage or entrapment when performing separation or fixation or when controlling bleeding, which may cause abnormal sensation in the nerve-innervated area, especially chronic neuropathic pain, and may even cause motor disorders in the lower extremity. It is extremely difficult to manage or improve these symptoms.
The iliopubic tract is a thickened tendinous structure of the transverse abdominal fascia that connects the anterior superior iliac spine and the pubic tubercle and parallels the inguinal ligament (Figure 13). It arches medially across the front of the femoral vessels to insert via broad attachment onto the pubic tubercle and Cooper’s ligament. The iliopubic tract is the outer boundary of the triangle of pain. The lateral part of a mesh should be fixated at a spot just above the level of the iliopubic tract. The white iliopubic tract can be seen at the lower edge of a direct hernia ring or below an internal inguinal ring. However, the degree of development of the iliopubic tract may vary individually; the iliopubic tract in other areas may not be easy to recognize under the laparoscope. The simplest method to identify the iliopubic tract is to touch and press the projected spot of the stapler head on the body surface when using a stapler to staple the lateral part of a mesh; the feel of the stapler head indicates that the stapler head is located above the iliopubic tract. Otherwise, the stapler head is likely located below the iliopubic tract, and stapling may cause nerve damage.
The vas deferens/the round ligament of the uterus and testicular blood vessels can be completely exposed only when the internal spermatic fascia is incised and the hernia sac or the peritoneal fold is separated to the cephalad direction.
Acknowledgements
Funding: This work was supported by the Shenzhen government funding for scientific and technical research and development (JCYJ20140414092023238).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Pisanu A, Podda M, Saba A, et al. Meta-analysis and review of prospective randomized trials comparing laparoscopic and Lichtenstein techniques in recurrent inguinal hernia repair. Hernia 2015;19:355-66. [Crossref] [PubMed]
- Pahwa HS, Kumar A, Agarwal P, et al. Current trends in laparoscopic groin hernia repair: A review. World J Clin Cases 2015;3:789-92. [Crossref] [PubMed]