The fetal liver as cell source for the regenerative medicine of liver and pancreas
Introduction
Recent advances on the anatomical arrangement of the liver parenchyma and biliary epithelium underlie the existences of multiple stem cell compartments composed of different stem/progenitor cells and related lineages (1). Particularly, fetal liver contains hepatic stem/progenitors cells within the ductal plates and multipotent stem/progenitor cells within large intrahepatic bile ducts and extrahepatic bile ducts (2-4). Moreover, both these niches are accompanied by mesenchymal companion cells, and the liver parenchyma contains a great number of other mesenchymal derived cells (e.g., macrophages, hematopoietic stem cells, endothelial, mesenchymal stem cells) (3). All of these different cell types have the peculiar characteristics as sources for cell therapy studies (5). Hepatocyte transplantation has been shown to be safe as cell therapy for liver diseases and patients showed clinical improvement with partial and transient correction of the underlying metabolic defects. The major challenges are the limited supply of donor organs, the quality of isolated cells and the low engraftment. The development of new technologies, particularly those based on stem/progenitor cells is in progress to overcome the shortage of organs (6). Islet transplantation is viewed as an ideal treatment for patients affected by type I diabetes mellitus, but it is constrained by the limited yield of quality donor pancreata that can be utilized to isolate islets (7). The hope is identify one or more precursor populations that can be lineage restricted to islet cells (8). The fetal liver offers the unique opportunity to isolate epithelial stem cells subpopulations with a wide spectrum of endodermal differentiation accompanied by a conspicuous mesenchymal cell population. Thus, it is the unique highly available and quality source contemporarily candidate for the regenerative medicine of liver and pancreas. This review deals with the different stem cell populations, isolable from fetal liver, candidate for cell therapy of liver diseases and diabetes and to discuss advantages and limitation with respect to other sources.
Embriology
From an embryological point of view, the liver shares a common origin with the ventral pancreas (9). A common stem⁄progenitor for liver, biliary epithelium, and pancreas exists at the earlier stages of development when the definitive anterior endoderm is forming the foregut (10-13). Within hours of endoderm formation, anterior endodermal epithelium folding generates the foregut and posterior folding creates the hindgut. After the activation of specific genetic programs for the development of liver or pancreas in endoderm cells, the endodermal epithelial cells develop a columnar shape (11). The hepatic and pancreatic endodermal cells then invade the local mesenchyme and form tissue buds. The signals regulating branch formation are not well understood but involve epithelial-mesenchymal signaling, along with mechanical forces and calcium signaling (14,15). The extrahepatic biliary epithelium originates directly from a portion of the ventral endoderm deriving from a pancreatobiliary stem⁄progenitor expressing PDX1 and SOX17 (16,17). The segregation of pancreatic and biliary precursors depends on SOX17; the primitive multipotent precursors are SOX17+⁄PDX1+ and give rise to SOX17+/PDX1- extrahepatic biliary cells and SOX17-/PDX1+ pancreatic cells (17). The endodermal proliferation occurring at the porta hepatis is thought to be the common phenomena giving rise to large (area and segmental) intrahepatic bile ducts. On the other hand, the formation of interlobular bile ducts and parenchymal cells (during the early phase of embryologic development) derives from the differentiation of human hepatic stem cells (hHpSCs) within the ductal plates, and their descendants, hepatoblasts, located close to the forming portal tract. Particularly the biliary differentiation is driven by the appearance of SOX9 expression (18-20).
Stem cell niches in adult liver
Stem cell niches within the liver are located in the canals of Hering. hHpSCs are precursors to hepatoblasts, which are presumed to be the transit-amplifying cells that first give rise to committed progenitors, and then to hepatocytes and cholangiocytes (21). Within the liver parenchyma, a maturational lineage exist from the stem cells in zone 1 (periportal), through the midacinar region (zone 2), to the most mature cells and apoptotic cells found pericentrally in zone 3 (3). Parenchymal cells are closely associated with lineages of mesenchymal cells, and their maturation is coordinated (22). Peribiliary glands (PBGs) contain stem cell niches within the biliary epithelium (1,4,23). These glands are along the biliary tree from the hepatopancreatic common duct near the duodenum up to the septal ducts (large intrahepatic bile ducts). High numbers of PBGs occur in the cystic duct, hilum and periampular regions, sites that are vulnerable to oncogenic transformation. Progenitor and/or stem cells within PBGs probably act as sources for cell turnover of the entire biliary tree distal to the interlobular bile ducts (1,4,23). This niche follows the model of the intestinal stem cell niche: proliferating cells and cells with a more primitive phenotype are located at the bottom of the gland, cells with an intermediate phenotype are placed in the middle of the gland, and fully differentiated cells are continuous with the surface epithelium. The endodermal stem⁄progenitor-like cells in the adult PBGs seem to be a remnant of endoderm and biliopancreatic progenitor cells that in fetal life determine the formation of the biliary tree (23).
Fetal liver tissues organization
The canals of Hering have long been proposed to be a stem cell niche in postnatal livers (2,18,24-27). The canals of Hering have been shown recently to derive from the ductal plates of fetal and neonatal livers (18). In these ductal plates, hHpSCs give rise to hepatoblasts, which are bi-potent progenitor cells able to differentiate in vivo and in vitro into mature hepatocytes and cholangiocytes (3,18,21,28). The fetal human biliary tree contains progenitor/stem cells of endodermal origin within the surface epithelium and bud of PBGs, which likely represent the fetal precursors to hBTSC populations persisting in the adult epithelium tree throughout life. Histology and immunohistochemical analysis revealed that the surface epithelium of extrahepatic and intrahepatic bile ducts and the bud of PBGs are mostly formed by EpCAM positive cells expressing PDX-1 and SOX17 that are transcription factors known to be essential for the formation of liver and pancreas.
Fetal liver cells isolation
Attempts to isolate and characterize fetal liver cells have been performed in the last years. Reid et al. firstly described the methods and the phenotypic characterization of stem/progenitor cells purified from livers of fetuses between 18-22 wks gestational age obtained by elective termination of pregnancy (2,29). All processing and cell enrichment procedures were conducted in basal medium supplemented with 0.1%, insulin and iron-saturated transferring and trace elements (selenious acid and ZnSO4). Liver tissue was mechanically disaggregated and subsequently digested in buffer containing type IV collagenase and deoxyribonuclease and finally filtered and before elimination of erythrocytes (2,29). This method allows the isolation of viable cells with a yield of approx. 1 billion cells per tissue donation (average of 3.8×107 cells per gram of tissue). A significant improved isolation technique has been recently developed by Gridelli et al. trough a 5-step cell isolation protocol based on vascular perfusion trough portal vein (30). Portal vein cannulation was surgically possible with fetuses no younger than a gestational age of 18 weeks. The in situ portal vein perfusion allows an exposure time to collagenase of less than 10 minutes instead of 40 minutes with conventional tissue fragment incubation (2,28). Vascular perfusion resulted in almost complete digestion of the connective tissue and better erythrocyte elimination. The cell suspensions obtained from these fetal livers contained, on average, 12% EpCAM+ cells (2,28,29). However, this percentage could be increased to 20% depending on the gestational age of the fetus. Most of the EpCAM negative cells in fetal livers were of non-hepatic lineage and were predominantly hematopoietic cells (2,29). The vast majority (90%) of the EpCAM positive cells in fetal livers coexpress α-fetoprotein (AFP), albumin, and cytokeratin-19 (CK19). EpCAM positive cells can be classified into two subpopulations: (I) hepatoblasts showing expression of ICAM1, AFP, albumin, CK-19, CD133/1, P450A7, and CD44H (hyaluronan receptor) (Figure 1), and (II) hHpSCs (Figure 1) positive for albumin (weak), CK19, CD44H, CD133/1, neural cell adhesion molecule (NCAM), and claudin 3, but negative for AFP and P450A7. Cell suspensions from adult human livers contain on average 1.3% EpCAM positive cells that increased 2-3 folds in neonatal and pediatric donors (2,29). As the gestational age increased, the percentage of CK-18/CK-19 immature bi-potential progenitors significantly decreased, and the percentage of mature hepatocytes increased. Interestingly, the percentage of fetal cells expressing proliferation markers was on average 45 times greater than the percentage of adult hepatocytes (30). The fetal liver (18th-22nd week) contains a relevant percentage of cells expressing markers associated with mesenchymal cells (Figure 1): 16.4%, 1.5%, and 7.6% of the cells were positive for vimentin, CD90 [thymus cell antigen 1 (Thy-1)], and CD29, respectively. Flow citometry (FC) demonstrated the presence of cells expressing hematopoietic progenitor markers: CD133 (2.2% of the cells), CD117 (c-Kit) as an early marker (1.2%), CD34 (4.1%), and CD45 as an overall white blood cell marker (6%). The percentage of Ki-67+ cells decreased as the gestational age increased (i.e., 69% at 18-19 weeks and 45% at 20-22 weeks) (30). Recently we studied the fetal biliary epithelium with the aim to isolate multipotent stem/progenitors cells using an approach previously used in the adult biliary tree (31). The intrahepatic biliary epithelium, comprising ducts distal to the second order branches, was isolated mechanically from liver parenchyma until a fine network of white ducts appeared. After the mechanical dissociation from liver parenchyma, both intrahepatic (large connective tissue tracts containing large IHBDs) and extrahepatic biliary tree specimens were enzymatically digested in buffer containing type I Collagenase and deoxyribonuclease. This procedure resulted in a homogeneous suspension of cell aggregates that were passed through an 80-micron mesh filter. After digestion of extrahepatic and intrahepatic biliary tree specimens, an average of 5 million and 16 million viable cells were isolated from extrahepatic and intrahepatic bile ducts, respectively. Almost all cells (>90%) expressed EpCAM and SOX17/PDX-1 at nuclear level and appeared negative for markers of mature liver cells [e.g., albumin, glutamyl transpeptidase (GGT), and secretin receptor (SR)] (31).
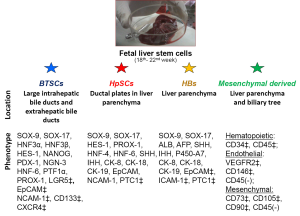
Resident stem cells populations in fetal liver
hHpSCs constitutes average 2% of the parenchyma of fetal livers. The hHpSCs cells range in size from 7-9 µm in diameter and have a high nucleus-to-cytoplasm ratio. The hHpSC phenotypic profile includes epithelial cell adhesion molecule (EpCAM), neural cell adhesion molecule (NCAM), CD133, CXCR4, SOX9, SOX17, FOXA2, cytokeratins (CK) 8/18/19, Hedgehog proteins (Sonic and Indian), intranuclear telomerase protein, claudin 3, MDR1, weak or negligible expression of albumin and major histocompatibility complex (MHC) antigens. Some proteins, such as CK-19 and albumin, are synthesized and found only in punctuate form, but not converted into filaments, or packaged, as in later lineage stages. In culture, the hHpSCs form colonies capable of self-replication (32) and of differentiation to mature liver cells (2,22). Cells expand ex vivo if cultured in Kubota’s medium, a serum-free medium containing only insulin, transferrin/fe, lipids, no copper, and low calcium (29,33), and if cocultured with angioblasts. hHpSCs showed in vivo in murine model of liver diseases a bipotential capability differentiation giving rise to mature cholangiocytes and hepatocytes. Hepatoblasts (hHBs) are diploid bipotent cells giving rise to hepatocytic and cholangiocytic lineages, associated with precursors of both endothelia and hepatic stellate cells, and the liver’s probable transit amplifying cells (21). Hepatoblasts can be isolated by dual immunoselection for EpCAM/ICAM-1 and have remarkable expansion potential cultured in Kubota’s medium. The hHBs, larger (10-12 µm) and with higher amounts of cytoplasm than hHpSCs, showed reduction in EpCAM levels, elevated albumin levels with discrete cytoplasmic packaging (2); switch from NCAM to ICAM-1, filamentous CK-14 and CK-19 (18,34,35), expression of early P450s (e.g., P450-A7) and CK-7, and strong positive expression of hepatic-specific AFP (3). The immunohistochemical analyses revealed that hBTSCs expressed surface markers (EpCAM, NCAM, CXCR4, Lgr5) and or transcription factors (FOXa2, SOX9, OCT4) typical of endodermal stem cells and progenitors. Cultures of expanding foetal human biliary stem progenitor cells (hBTSCs) are also obtained from fetal tissue plated onto plastic and in serum-free KM. In these conditions, the foetal hBTSCs remained phenotypically stable. Each colony was formed by small (diameter =9.84 µm), densely packed and uniform cells with high nucleus to cytoplasmic ratio. Almost all cells (>90%) expressed EpCAM and SOX17/PDX-1 at nuclear level; cell cultures were constantly negative for markers of mature liver cells (e.g., albumin, GGT and SR). Given their embryological origins, hBTSCs are logical precursors for mature cells of liver, bile ducts, and pancreas. In vivo studies, consisted of fetal hBTSCs transplantation into the livers of SCID mice, demonstrated that fetal hBTSCs engrafted into the livers and differentiated towards human hepatocytes. Moreover, hBTSCs differentiated towards mature cholangiocytes, having been individuated within the canals of Hering and interlobular bile ducts. The data show that fetal hBTSCs are multipotent and have biological properties comparable to that observed in hBTSCs isolated from adult biliary tissue (4). However, further in vitro and in vivo studies will be necessary to test if fetal hBTSCs could have a higher plasticity in comparison with adult ones. The hBTSCs from foetal tissue could, theoretically, be considered as an ideal source for cell therapies of liver and pancreas. The possibility to form pancreatic islets from hepatic progenitors has been also studied in adult mice. This study showed that hepatic progenitor cells can self assemble to form 3D islet-like clusters that express pancreatic islet cell differentiation-related transcripts and islet-specific hormones (36). The islet-like structures derived from these progenitor cells have the ability to reverse hyperglycemia in non-obese diabetic mice with severe combined immunodeficiency. These results have been interpreted as an expression of the differentiation plasticity of hepatic progenitor cells. However, on the light of the recent discovery concerning the existence of multipotent stem cells within the intrahepatic biliary tree, an alternative explanation is that the hepatic progenitor cell population includes a subpopulation of multipotent stem/progenitor cell that originate from the PBGs and that are capable of differentiation into pancreatic tissue (1). Indeed, we have recently tested the ability of human hepatic progenitor cells to differentiate towards pancreatic fate: no islet like structures have been observed in these experimental setting. Stem and progenitor cells committed to hematopoietic, endothelial, epithelial, and mesenchymal lineages were clearly identifiable in livers. These stem cells and progenitors are recognizable trough their specific phenotype profiles: hematopoietic [CD34(+)/CD45(+)], endothelial [vascular endothelial growth factor-2 (KDR)(+)/CD146(+)/CD45(-)], mesenchymal [CD73(+)/CD105(+)/CD90 (Thy-1)(+)/CD45 (-)] (37). Within the human liver, c-kit+ pluripotent hematopoietic stem cells, residing in the perisinusoidal Disse spaces, give rise to all lineages of leukocytes and red blood cells (38-41). These mesenchymal derived liver mononuclear cells can reconstitute the thymus, liver leukocytes, splenocytes, lymph nodes bone marrow cells in lethally irradiated B cell- and T cell-deficient SCID mice (42). There are several advantages of co-isolating hBTSCs from foetal specimens in comparison with other procedures that normally discard the biliary tree. Collecting the extrahepatic biliary tree from the foetus and performing a parallel isolation of both hBTSCs and hepatic progenitor cells (hHBs and hHpSCs) from the fetal liver results in an increase in cell yield and in the isolation of a cell population with a wider differentiative potential.
Isolation of clinical grade fetal liver cells
Fetal liver cells isolation can be carried out under GMP (good manufacturing practices) conditions. Indeed, in several studies, mothers underwent to assessment of infectious diseases (HCV, HBV, HIV, EBV, HEV, HDV, toxoplasmosis, rubella, cytomegalovirus, parvovirus, herpes simplex type 1 and 2, TPHA) as required by current regulation. The production of the cell is possible according to “The rules governing medicinal products in the European Union” and the European guidelines of GMP for medicinal products for human use (EudraLex - Volume 4 Good manufacturing practice Guidelines). Indeed, all media are disposable procured as “cGMP-manufactured” from commercial distributors. Sterility testing and endotoxin testing are readily highly available. EpCAM sorting procedure is highly standardized and has been already used in program of human cell therapy without complication (43). Recently EpCAM expression has been demonstrated to mark hepatic stem/progenitors cells (2), biliary tree stem/progenitor cells and their committed progenies (4,23,31). Newly derived EpCAM positive hepatocytes have been observed within the human hepatic lobules (44). Cholangiocytes with a committed phenotype (e.g., goblet cells or mature cholangiocytes) showed EpCAM and PAS expression (23). EpCAM positive cells from PBGs showed a multipotent endodermal differentiation capability (4). Thus, EpCAM isolation determines the enrichment of populations of stem/progenitor cells, together with their uni-potential committed progeny.
Clinical results in liver cell therapy with fetal hepatic stem/progenitor cells
Fetal liver cells may be therapeutically useful for a variety of cholestatic liver diseases. This includes a number of genetic/metabolic liver diseases, acute liver failure, and liver cirrhosis. In recent studies, isolated hepatic progenitor cells (hepatoblasts and hHpSCs) from fetal liver were used to cure lethal congenital liver diseases. By infusing through the hepatic artery the equivalent of 5% of parenchymal mass of hepatocytes in a patient with Crigler–Najjar syndrome and one with biliary atresia, a significant improvement of all liver serum biochemistry has been achieved (45,46). Second-trimester fetal liver cells, enriched of EpCAM + cells and labeled with Tcd, l-hexamethyl-propylene-amine oxime (Tc-HMPAO) were infused into the hepatic artery of 25 patients with end stage liver cirrhosis. A marked improvement of clinical and biochemical parameters has been observed and the mean Model for End-Stage Liver Disease (MELD) score decreased, during 6 months follow-up, in all patients. The cells restored the lost liver function in the patients without provoking an immune reaction, probably because of the absence of HLA class II expression of the infused cells (43). Interestingly, by the use of magnetic nuclear imaging techniques, engraftment of the injected cells in the liver has been demonstrated. Therapy using human fetal liver stem/progenitor cells in advanced cirrhosis offers a potentially supportive modality of bridging to organ transplantation. Fetal stem/progenitor cells could also be ideal vehicles for delivering therapeutic genes into the liver (47,48). Finally, they can readily be isolated and would not require immunosuppression (2).
Advantages of fetal liver with respect to other cell sources for the cell therapy of liver diseases
One of the main hindrances of hepatocyte transplantation is the limited source and marginal quality of cells obtained from adult human livers (6,49). Bone marrow is another source of stem/progenitor cells (50). In general, clinical trials using autologous bone marrow derived stem cells provided interesting data regarding the safety and some short-term benefit in the treatment of chronic liver disease (51). However, the mechanism of action of bone marrow-derived stem cells in the setting of liver disease treatment is unclear (50). Embryonic stem cells are able to proliferate in an unlimited fashion and produce large numbers of hepatocyte-like cells (52,53). In vitro, these cells have been shown to have reasonable functional capacity (54), although there is still caution about their use for transplantation due to their propensity to form both malignant and non-malignant tumors (55). Nevertheless, the development of cell replacement therapies using embryonic stem (ES) cells is, however, hampered by ethical concerns as well as issues involving immune rejection of the transplanted cells (55). A recent development allows the production of similar cells, induced pluripotent stem cells (iPSCs), by over-expressing transcription factors such as SOX2 and Oct4 in adult somatic cells. Hepatocytes derived from iPSCs have reasonable synthetic and metabolic capacity, and seem to be similar to cells derived from ES cells (5,55-57). The ability to generate iPSCs by direct reprogramming of human fibroblasts has brought the realization of personalized regenerative medicines closer. Personalized iPSCs tailored to specific individuals should provide the opportunity for autologous cell replacement therapy without the need of immunosuppression (55). However, concerns remain about their use in a transplant setting, as we cannot yet be certain that these cells would not undergo reversion to more primitive state with uncontrolled tumorigenic expansion within the recipient. Moreover, other obstacles are linked with important technical issues, such as increasing the efficiencies of iPSCs generation using non-integrating methods, as well as creating them under cGMP conditions (55). In this context, the large availability of fetal livers and their biological properties, comprising scarce or null immunogenicity and tumoregenicity (2,31,58,59), guaranty an ideal source of easily isolable and cultivable stem cells. Indeed, clinical trials with hHpSCs from foetal livers have been successfully completed and did not require immunosuppression of the recipients (58).
New horizons in liver cell therapy
There is an increasing range of potential applications of stem cells in liver diseases, with many clinical studies already undertaken. In genetic/metabolic liver diseases (Ornithine transcarbamylase deficiency, Glycogen Storage disease 1a, Familial Hypercholesterolemia, Crigler-Najjar type I, factor VII deficiency, PFIC), the main objective is to replace the missing function, in acute liver failure is the recovery and regeneration of the injured native liver, in chronic or acute-on-chronic liver disease is to rescue liver injury and function. The different available cell sources should be tailored for the specific pathological conditions according the specific and peculiar properties they are endowed with. Indeed, hematopoietic stem cells and macrophages resulted particularly suitable to improve regeneration and reduce scarring in liver cirrhosis by modulating the liver’s own regenerative processes, mesenchymal stem cells posses the capability to down-regulate immune mediated liver damage and, finally, hepatoblasts, hHpSCs, hBTSCs, iPSCs and ES cells are the suitable sources to supply hepatocyte-like cells (5). Fetal liver offers the unique opportunity to isolate both epithelial stem cells populations and a conspicuous mesenchymal cell population. Mesenchymal cells (hematopoietic, endothelial, mesenchymal stem cells, and macrophages) (2,30,37,42) within the fetal liver are furnished with high value biology properties, comprising fibrosis remodeling and immune-modulation. Moreover, epithelial and their mesenchymal companion cells showed already a positive synergism on ex vivo expansion (2). A cell therapy with fetal liver cells could determine wide and profound beneficial effects in the diseased human liver, as expected for a (cell-) biological therapy. Indeed, in cirrhotic liver this epithelial/mesenchymal synergism could be highly effective both in fibrosis remodelling and liver repopulation. As a confirmation of this hypothesis the first cirrhotic patient transplanted with the whole fetal liver cells (epithelial and mesenchymal cells) showed MELD score improvement from 15 to 10 within the first 18 months of observation (30).
Potential role of fetal btscs in pancreas regenerative medicine
Islet transplantation is viewed as an ideal treatment for type I diabetes, but it is constrained by the limited yield of quality donor pancreata that can be used to isolate islets (7). Recently, a new source of islet precursors has been identified in the biliary epithelium of donors of all ages (4). It is noteworthy that lineage restriction of ES cells, iPSCs or mesenchymal stem cells (MSCs) to an islet fate requires transfection with key transcription factors (e.g., PDX1) and sequential treatments with a panel of growth factors and matrix components for 4 to 6 weeks (60,61). By contrast, the biliary stem cells are already at the stage 4 of the 5 stages of the differentiation process previously described (62) and are poised to generate islets rapidly in an appropriate microenvironment and without gene manipulation. Given the assumption that pancreatic stem cells are lacking in postnatal tissues, a number of cell sources have been studied including: ES cells (59,60,63), iPSCs (64,65), MSCs from bone marrow or cord blood (66), adipose tissue or amniotic fluid-derived stem cells (67,68), transdifferentiation of acinar cells to islet cells by genetic manipulations (69,70). Although these studies are producing a wealth of information regarding pancreatic development and functions, they will not lead to clinical programs until a number of challenges are overcome. In contrast to these different proposals, the biliary tree, in both fetal and adult life is replete with large numbers of multipotent stem cells (1,4,23,31). The ready availability of biliary tree tissues from fetal liver and the extensive expansion potential of the biliary tree stem cells in culture under wholly defined conditions makes biliary tree stem cells a viable option for clinical programs in the treatment of diabetes and other pancreatic diseases.
Conclusions
Recent advances indicate the fetal biliary tree as an ideal cell source of hepato-pancreatic stem/progenitor cells. In comparison with hHpSCs and hepatoblasts, hBTSCs showed multipotent capabilities and there selective isolation would be considered to obtain differentiated mature hepatocytes, cholangiocytes and beta-pancreatic cells. In addition, the fetal liver posses a unique feature given the co-existence of endodermal and mesenchymal derived cells. Therefore, it is the unique highly available source candidate contemporarily for the regenerative medicine of liver and pancreas, and hypothetically the more useful and qualified to address the main areas (fibrosis remodelling and liver repopulation) requested for an effective cure of liver cirrhosis.
Acknowledgements
E. Gaudio was supported by research project grant from the University “Sapienza” of Rome and FIRB grant # RBAP10Z7FS_001 and by PRIN grant # 2009X84L84_001. D. Alvaro was supported by FIRB grant # RBAP10Z7FS_004 and by PRIN grant # 2009X84L84_002. V. Cardinale was supported by FIRB grant # RBAP10Z7FS_004. The study was also supported by Consorzio Interuniversitario Trapianti d’Organo, Rome, Italy. A special thank to Prof. Maurizio Anceschi for him great collaborative efforts in the studies carried in Sapienza University of Rome on fetal liver.
Disclosure: The authors declare no conflict of interest.
References
- Cardinale V, Wang Y, Carpino G, et al. The biliary tree--a reservoir of multipotent stem cells. Nat Rev Gastroenterol Hepatol 2012;9:231-40. [PubMed]
- Schmelzer E, Zhang L, Bruce A, et al. Human hepatic stem cells from fetal and postnatal donors. J Exp Med 2007;204:1973-87. [PubMed]
- Turner R, Lozoya O, Wang Y, et al. Human hepatic stem cell and maturational liver lineage biology. Hepatology 2011;53:1035-45. [PubMed]
- Cardinale V, Wang Y, Carpino G, et al. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology 2011;54:2159-72. [PubMed]
- Forbes SJ, Newsome PN. New horizons for stem cell therapy in liver disease. J Hepatol 2012;56:496-9. [PubMed]
- Puppi J, Strom SC, Hughes RD, et al. Improving the techniques for human hepatocyte transplantation: report from a consensus meeting in London. Cell Transplant 2012;21:1-10. [PubMed]
- Mineo D, Pileggi A, Alejandro R, et al. Point: Steady Progress and Current Challenges in Clinical Islet Transplantation. Diabetes Care 2009;32:1563-69. [PubMed]
- Aguayo-Mazzucato C, Bonner-Weir S. Stem cell therapy for type 1 diabetes mellitus. Nat Rev Endocrinol 2010;6:139-48. [PubMed]
- Nakanuma Y. A novel approach to biliary tract pathology based on similarities to pancreatic counterparts: is the biliary tract an incomplete pancreas? Pathol Int 2010;60:419-29. [PubMed]
- Lemaigre FP. Mechanisms of liver development: concepts for understanding liver disorders and design of novel therapies. Gastroenterology 2009;137:62-79. [PubMed]
- Wandzioch E, Zaret KS. Dynamic signaling network for the specification of embryonic pancreas and liver progenitors. Science 2009;324:1707-10. [PubMed]
- Si-Tayeb K, Lemaigre FP, Duncan SA. Organogenesis and development of the liver. Dev Cell 2010;18:175-89. [PubMed]
- Zong Y, Stanger BZ. Molecular mechanisms of bile duct development. Int J Biochem Cell Biol 2011;43:257-64. [PubMed]
- Rochefort NL, Konnerth A. Genetically encoded calcium sensors come of age. Nat Methods 2008;5:761-62. [PubMed]
- Beloussov LV, Gordon R. Preface: Developmental morphodynamics - bridging the gap between the genome and embryo physics. Int J Dev Biol 2006;50:79-80.
- Roskams T, Desmet V. Embryology of extra- and intrahepatic bile ducts, the ductal plate. Anat Rec (Hoboken) 2008;291:628-35. [PubMed]
- Spence JR, Lange AW, Lin SC, et al. Sox17 regulates organ lineage segregation of ventral foregut progenitor cells. Dev Cell 2009;17:62-74. [PubMed]
- Zhang L, Theise N, Chua M, et al. The stem cell niche of human livers: symmetry between development and regeneration. Hepatology 2008;48:1598-607. [PubMed]
- Antoniou A, Raynaud P, Cordi S, et al. Intrahepatic bile ducts develop according to a new mode of tubulogenesis regulated by the transcription factor SOX9. Gastroenterology 2009;136:2325-33. [PubMed]
- Furuyama K, Kawaguchi Y, Akiyama H, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet 2011;43:34-41. [PubMed]
- Wang Y, Yao HL, Cui CB, et al. Paracrine signals from mesenchymal cell populations govern the expansion and differentiation of human hepatic stem cells to adult liver fates. Hepatology 2010;52:1443-54. [PubMed]
- Wang Y, Cui CB, Yamauchi M, et al. Lineage restriction of human hepatic stem cells to mature fates is made efficient by tissue-specific biomatrix scaffolds. Hepatology 2011;53:293-305. [PubMed]
- Carpino G, Cardinale V, Onori P, et al. Biliary tree stem/progenitor cells in glands of extrahepatic and intraheptic bile ducts: an anatomical in situ study yielding evidence of maturational lineages. J Anat 2012;220:186-99. [PubMed]
- Alison MR, Golding MH, Sarraf CE, et al. Pluripotential liver stem cells: facultative stem cells located in the biliary tree. Cell Prolif 1996;29:373-402. [PubMed]
- Gaudio E, Carpino G, Cardinale V, et al. New insights into liver stem cells. Dig Liver Dis 2009;41:455-62. [PubMed]
- Okabe M, Tsukahara Y, Tanaka M, et al. Potential hepatic stem cells reside in EpCAM+ cells of normal and injured mouse liver. Development 2009;136:1951-60. [PubMed]
- Spee B, Carpino G, Schotanus BA, et al. Characterisation of the liver progenitor cell niche in liver diseases: potential involvement of Wnt and Notch signalling. Gut 2010;59:247-57. [PubMed]
- Schmelzer E, Wauthier E, Reid LM. The phenotypes of pluripotent human hepatic progenitors. Stem Cells 2006;24:1852-8. [PubMed]
- Wauthier E, Schmelzer E, Turner W, et al. Hepatic stem cells and hepatoblasts: identification, isolation, and ex vivo maintenance. Methods Cell Biol 2008;86:137-225. [PubMed]
- Gridelli B, Vizzini G, Pietrosi G, et al. Efficient human fetal liver cell isolation protocol based on vascular perfusion for liver cell-based therapy and case report on cell transplantation. Liver Transpl 2012;18:226-37. [PubMed]
- Semeraro R, Carpino G, Cardinale V, et al. Multipotent stem/progenitor cells in the human foetal biliary tree. J Hepatol 2012;57:987-94. [PubMed]
- McClelland R, Wauthier E, Zhang L, et al. Ex vivo conditions for self-replication of human hepatic stem cells. Tissue Eng Part C Methods 2008;14:341-51. [PubMed]
- Kubota H, Reid LM. Clonogenic hepatoblasts, common precursors for hepatocytic and biliary lineages, are lacking classical major histocompatibility complex class I antigen. Proc Natl Acad Sci U S A 2000;97:12132-7. [PubMed]
- Kuwahara R, Kofman AV, Landis CS, et al. The hepatic stem cell niche: identification by label-retaining cell assay. Hepatology 2008;47:1994-2002. [PubMed]
- Haruna Y, Saito K, Spaulding S, et al. Identification of bipotential progenitor cells in human liver development. Hepatology 1996;23:476-81. [PubMed]
- Yang L, Li S, Hatch H, et al. In vitro trans-differentiation of adult hepatic stem cells into pancreatic endocrine hormone-producing cells. Proc Natl Acad Sci U S A 2002;99:8078-83. [PubMed]
- Porretti L, Cattaneo A, Colombo F, et al. Simultaneous characterization of progenitor cell compartments in adult human liver. Cytometry A 2010;77:31-40. [PubMed]
- Watanabe Y, Morita M, Akaike T. Concanavalin A induces perforin-mediated but not Fas-mediated hepatic injury. Hepatology 1996;24:702-10. [PubMed]
- Taniguchi H, Masuyama M, Koyama H, et al. Quantitative measurement of human tissue hepatic blood volume by C15O inhalation with positron-emission tomography. Liver 1996;16:258-62. [PubMed]
- Seki S, Sakaguchi H, Kitada T, et al. Outcomes of dysplastic nodules in human cirrhotic liver: a clinicopathological study. Clin Cancer Res 2000;6:3469-73. [PubMed]
- Crosbie OM, Reynolds M, McEntee G, et al. In vitro evidence for the presence of hematopoietic stem cells in the adult human liver. Hepatology 1999;29:1193-8. [PubMed]
- Seki S, Habu Y, Kawamura T, Takeda K, et al. The liver as a crucial organ in the first line of host defense: the roles of Kupffer cells, natural killer (NK) cells and NK1.1 Ag+ T cells in T helper 1 immune responses. Immunol Rev 2000;174:35-46. [PubMed]
- Khan AA, Shaik MV, Parveen N, et al. Human fetal liver-derived stem cell transplantation as supportive modality in the management of end-stage decompensated liver cirrhosis. Cell Transplant 2010;19:409-18. [PubMed]
- Yoon SM, Gerasimidou D, Kuwahara R, et al. Epithelial cell adhesion molecule (EpCAM) marks hepatocytes newly derived from stem / progenitor cells in humans. Hepatology 2011;53:964-73. [PubMed]
- Khan AA, Parveen N, Mahaboob VS, et al. Treatment of Crigler-Najjar Syndrome type 1 by hepatic progenitor cell transplantation: a simple procedure for management of hyperbilirubinemia. Transplant Proc 2008;40:1148-50. [PubMed]
- Khan AA, Parveen N, Mahaboob VS, et al. Management of hyperbilirubinemia in biliary atresia by hepatic progenitor cell transplantation through hepatic artery: a case report. Transplant Proc 2008;40:1153-5. [PubMed]
- Yusa K, Rashid ST, Strick-Marchand H, et al. Targeted gene correction of α1-antitrypsin deficiency in induced pluripotent stem cells. Nature 2011;478:391-4. [PubMed]
- Sandhaus RA. Gene therapy meets stem cells. N Engl J Med 2012;366:567-9. [PubMed]
- Dhawan A, Puppi J, Hughes RD, et al. Human hepatocyte transplantation: current experience and future challenges. Nat Rev Gastroenterol Hepatol 2010;7:288-98. [PubMed]
- Houlihan DD, Newsome PN. Critical review of clinical trials of bone marrow stem cells in liver disease. Gastroenterology 2008;135:438-50. [PubMed]
- Peng L, Xie DY, Lin BL, et al. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology 2011;54:820-8. [PubMed]
- Woo DH, Kim SK, Lim HJ, et al. Direct and indirect contribution of human embryonic stem cell-derived hepatocyte-like cells to liver repair in mice. Gastroenterology 2012;142:602-11. [PubMed]
- Hay DC, Fletcher J, Payne C, et al. Highly efficient differentiation of hESCs to functional hepatic endoderm requires ActivinA and Wnt3a signaling. Proc Natl Acad Sci USA 2008;105:12301-6. [PubMed]
- Brolén G, Sivertsson L, Bjorquist P, et al. Hepatocyte-like cells derived from human embryonic stem cells specifically via definitive endoderm and a progenitor stage. J Biotechnol 2010;145:284-94. [PubMed]
- Kiskinis E, Eggan K. Progress toward the clinical application of patient-specific pluripotent stem cells. J Clin Invest 2010;120:51-9. [PubMed]
- Sancho-Bru P, Roelandt P, Narain N, et al. Directed differentiation of murine-induced pluripotent stem cells to functional hepatocyte-like cells. J Hepatol 2011;54:98-107. [PubMed]
- Sullivan GJ, Hay DC, Park IH, et al. Generation of functional human hepatic endodermfrom human induced pluripotent stem cells. Hepatology 2010;51:329-35. [PubMed]
- Khan MR, Marium A, Shabbir M, et al. Antioxidant and hepatoprotective effects of Oxalis corniculata against carbon tetrachloride (CCl4) induced injuries in rat. African J Pharmacy Pharmacol 2012;6:2255-67.
- Parasassi T, Brunelli R, Bracci-Laudiero L, et al. Differentiation of normal and cancer cells induced by sulfhydryl reduction: biochemical and molecular mechanisms. Cell Death Differ 2005;12:1285-96. [PubMed]
- D'Amour KA, Agulnick AD, Eliazer S, et al. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol 2005;23:1534-41. [PubMed]
- Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 2008;26:443-52. [PubMed]
- Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 2008;132:661-80. [PubMed]
- D’Amour KA, Bang AG, Eliazer S, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nature Biotechnology 2006;24:1392-401. [PubMed]
- Ku HT, Zhang N, Kubo A, et al. Committing embryonic stem cells to early endocrine pancreas in vitro. Stem Cells 2004;22:1205-17. [PubMed]
- Zhang L, Wu HS, Chen Y, et al. Role of nitric oxide in Toll-like receptor 2 and 4 mRNA expression in liver of acute hemorrhagic necrotizing pancreatitis rats. World J Gastroenterol 2006;12:485-8. [PubMed]
- Prabakar KR, Dominguez-Bendala J, Molano RD, et al. Generation of glucose-responsive, insulin-producing cells from human umbilical cord blood-derived mesenchymal stem cells. Cell Transplant 2012;21:1321-39. [PubMed]
- De Coppi P, Bartsch G, Siddiqui MM, et al. Isolation of amniotic stem cell lines with potential for therapy. Nature Biotechnology 2007;25:100-6. [PubMed]
- Furth ME, Atala A. Stem cell sources to treat diabetes. J Cell Biochem 2009;106:507-11. [PubMed]
- Zhou Q, Brown J, Kanaredk A, et al. In vivo reprogramming of adult pancreatic exocrine cells to beta cells. Nature 2008;455:627-32. [PubMed]
- Zhou Q, Melton DA. Extreme makeover: converting one cell into another. Cell Stem Cell 2008;3:382-8. [PubMed]


