Critical laboratory values communication: summary recommendations from available guidelines
Laboratory diagnostics is critical to both the clinical decision making and to the managed care of the vast majority of human disorders (1). Quality testing encompasses a number of aspects spanning throughout the total testing process, and hence beginning from test ordering and ideally concluding with results communication to the requesting physician. Despite several lines of evidence attest that the vast majority of diagnostic errors emerge from the so-called preanalytical phase (2), sample analysis and transmission of test results are also vulnerable parts of the total testing process. As regards the last aspect, the identification and timely communication of “highly pathological” values are still regarded as essential elements of good laboratory practice (3).
The appropriate definition of highly pathological (also known as “alert” or “panic”) values has challenged the minds of many health care managers, physicians and laboratory professionals for decades (4). Several concepts have been developed, some of which partially overlapping but likewise presenting notable peculiarities. The very first approach to this issue has been provided by Lundberg more than 40 years ago (5), and has then been reiterated and refined by many international and national organizations in the following years. The Joint Commission (JC), an independent and not-for-profit organization endeavored to improve patient safety and quality of health care, defines a critical test result as “a test that requires immediate communication of result irrespective of whether it is normal, significantly abnormal or critical” (6). This definition is also shared by many other organizations such as the Clinical and Laboratory Standards Institute (CLSI) (7) and the Royal College of Pathologists (RCP) (8). Critical value is instead defined by the JC as “a test result that is significantly outside the normal range and may represent life-threatening values” (6). This designation is quite similar to the concept of critical risk result endorsed by the RCP (i.e., “a test result that is life threatening, or indicates significant morbidity or irreversible harm if immediate medical action is not taken”) (8). A significant risk result is finally defined by the JC as “a test result that is not life threatening but requires timely medical attention and follow-up action within a medically justified timescale” (6).
Although a certain agreement seemingly exists among the various national and international organizations for defining the clinical significance of critical values, several lines of evidence suggest that the policies for implementation of their communication are dramatically heterogeneous around the globe. The results of surveys conducted in the UK (9), Italy (10), US (11), China (12) and Croatia (13) have notably emphasized that there is poor consensus regarding many aspects of critical values management. This is a rather concerning issue, for not less than three good reasons. First, the lack or delayed communication of critical values has been clearly recognized as a source of significant harm to the patients (14), since these test results may led to treatment modification in as many as 98% of patients admitted to surgical wards and up to 91% of those admitted to medical departments (15). Then, critical values communication is now an integral part of many accreditation procedures for medical laboratories, including the universally agreed International Organization for Standardization (ISO) 15189:2012 (16). Finally, timely notification of critical values has been endorsed as one of the leading quality indicators of the post-analytical phase by the Working Group “Laboratory Errors and Patient Safety” (WG-LEPS) of the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) (17).
Despite we would all agree that the timely and efficient communication of critical laboratory values is an unavoidable part of managed care and patient safety, worldwide harmonization of practices is expected to be a rather long and winding road. A comprehensive search of current scientific resources yields not less than four recent and largely used documents (6-8,18). Notably, although some key concepts are basically shared by all these recommendations (e.g., especially the mandatory need to implement practices of communicating critical values and notification recording), there are many additional indications that are not really harmonized, and these especially regard which parameters (and the relative alarm values) should be included in the list of critical values, the time limits for notification, as well as to whom, how and by who critical values should be communicated. A detailed description of the various guidance is provided in Table 1. An ample consensus can be reached for some of these aspects, namely the time limits (i.e., critical values should be generally notified within 1 hour from their identification) and to whom they should be released (i.e., the responsible caregiver, by following a detailed escalation process), whereas the list of tests, the alarm values, the complete information that should be communicated as well as the details of the recording procedure cannot be thoughtfully combined. In an additional effort to generate a reliable guidance by integrating and transposing the most important aspects of each document, Table 1 also provides some “summary recommendations”, which are meant to depict the possible best laboratory practice derived from available consensus indications.
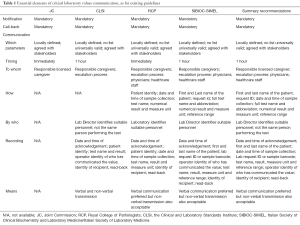
Full table
Information technology is increasingly becoming an essential component of medical laboratories, thus unraveling interesting perspectives also for the urgent communication with the clinics. Despite verbal communication has been for long considered the preferred procedure for notifying critical values, emerging non-verbal means of transmission may also be acceptable (19), provided that some essential criteria are fulfilled (e.g., timeliness of reporting, monitoring the impact of automated systems on clinical actions, verifying the correct system operation in various downtime scenarios, preliminary agreement with all stakeholders of laboratory services).
The efficient and timely communication of laboratory test results needing urgent clinical decision making is an essential responsibility of medical laboratories in order to optimizing the clinical management and lowering the risk of patient harm. Nonetheless, the many available documents about this essential aspect of patient care call for urgent and compelling harmonization of existing policies around the globe. We do hope that the “summary recommendations” provided in Table 1 may represent a reasonable basis for developing a widespread consensus.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lippi G, Plebani M, Graber ML. Building a bridge to safe diagnosis in health care. The role of the clinical laboratory. Clin Chem Lab Med 2016;54:1-3. [Crossref] [PubMed]
- Lippi G, Banfi G, Church S, et al. Preanalytical quality improvement. In pursuit of harmony, on behalf of European Federation for Clinical Chemistry and Laboratory Medicine (EFLM) Working group for Preanalytical Phase (WG-PRE). Clin Chem Lab Med 2015;53:357-70. [Crossref] [PubMed]
- Plebani M, Lippi G. Improving the post-analytical phase. Clin Chem Lab Med 2010;48:435-6. [Crossref] [PubMed]
- Piva E, Plebani M. From "panic" to "critical" values: which path toward harmonization? Clin Chem Lab Med 2013;51:2069-71. [Crossref] [PubMed]
- Lundberg G. When to panic over an abnormal value. Med Lab Obs 1972;4:47-54.
- Joint Commission. 2016 National Patient Safety Goals 2016. Available online: https://www.jointcommission.org/hap_2016_npsgs/, accessed 22 September 2016.
- Clinical and Laboratory Standards Institute (CLSI). Management of Critical- and Significant-Risk Results, 1st Edition. CLSI guideline GP47. Available online: http://shop.clsi.org/c.1253739/site/Sample_pdf/GP47Ed1_sample.pdf
- Campbell C, Caldwell G, Coates P, et al. Consensus Statement for the Management and Communication of High Risk Laboratory Results. Clin Biochem Rev 2015;36:97-105. [PubMed]
- Tillman J, Barth JH, ACB National Audit Group. A survey of laboratory 'critical (alert) limits' in the UK. Ann Clin Biochem 2003;40:181-4. [Crossref] [PubMed]
- Lippi G, Giavarina D, Montagnana M, et al. National survey on critical values reporting in a cohort of Italian laboratories. Clin Chem Lab Med 2007;45:1411-3. [Crossref] [PubMed]
- Wagar EA, Friedberg RC, Souers R, et al. Critical values comparison: a College of American Pathologists Q-Probes survey of 163 clinical laboratories. Arch Pathol Lab Med 2007;131:1769-75. [PubMed]
- Zeng R, Wang W, Wang Z. National survey on critical values notification of 599 institutions in China. Clin Chem Lab Med 2013;51:2099-107. [Crossref] [PubMed]
- Kopcinovic LM, Trifunović J, Pavosevic T, et al. Croatian survey on critical results reporting. Biochem Med (Zagreb) 2015;25:193-202. [Crossref] [PubMed]
- Plebani M, Carraro P. Mistakes in a stat laboratory: types and frequency. Clin Chem 1997;43:1348-51. [PubMed]
- Piva E, Pelloso M, Penello L, et al. Laboratory critical values: automated notification supports effective clinical decision making. Clin Biochem 2014;47:1163-8. [Crossref] [PubMed]
- International Organization for Standardization. ISO 15189:2007. Medical laboratories: Particular requirements for quality and competence. Available online: http://www.iso.org/iso/catalogue_detail?csnumber=42641
- Sciacovelli L, Aita A, Padoan A, et al. Performance criteria and quality indicators for the post-analytical phase. Clin Chem Lab Med 2016;54:1169-76. [Crossref] [PubMed]
- Lippi G, Caputo M, Banfi G, et al. Recommendations for the detection and management of critical values in clinical laboratories. Biochim Clin 2008;32:209-16.
- Piva E, Lippi G, Plebani M. Notification of abnormal and critical values: the road ahead. Am J Med 2010;123:e19; author reply e21.


