Treatment strategies according to genotype for chronic hepatitis B in children
Introduction
Most children experience a period of immune-tolerance during late childhood or adolescence, and some researchers assert that this is the main reason why treatment for hepatitis B virus (HBV) infected children is not indicated (1). However, in Asia, there are already some reports about hepatocellular carcinoma (HCC) in HBV infected children. Additionally, after experiencing severe hepatitis during the immune-clearance phase for a long period, the risk of HCC is higher in Asian children than in western children. Therefore, early detection of active hepatitis by regularly monitoring HBV infected children is essential and suitable treatment options must be employed. The choice of therapy is determined by the district (Western/Eastern), HBV genotype, medical accessibility, and economic state of the country. In this review, we intended to introduce updated information about pediatric chronic hepatitis B (CHB) infections according to differences in genotype and help physicians treat HBV infected children.
Selection of patients for treatment
Treatment indication
The main objective of CHB treatment is diminishing the risk of complications related to chronic liver disease. The therapeutic indications for both HBeAg-positive and HBeAg-negative CHB are generally the same as adults. The three main indications are serum HBV DNA levels, serum ALT levels, and the severity of liver disease (determined by liver biopsy or other less invasive tools). Practitioners also have to consider age, physical condition, and family history of HCC or liver cirrhosis (2,3).
In HBeAg-positive individuals, normal ALT levels, minimal or no liver inflammation determined by biopsy, and elevated serum HBV DNA levels are the characteristics of the immune-tolerance phase. The immune-clearance phase is characterized by a positive HBeAg state, low serum HBV DNA levels, high ALT levels, a moderate or severe hepatic inflammation state, and accelerated fibrosis. The treatment indication for CHB includes HBV DNA levels above 20,000 IU/mL, pretreatment serum ALT levels above 2 folds of the upper limit of normal (ULN) ALT levels for 6 months, and/or liver disease determined by liver biopsy showing an active hepatitis state.
The immune-tolerance phase is influenced by several factors. A younger age at diagnosis, vertical infection, suppressed immune status, Asian ethnicity, viral genotype (D > A, C > B), and high histological and biochemical activity are strongly related to a longer immune-tolerance phase (4).
Activation to active hepatitis B
The duration of the immune-tolerance phase is unpredictable. In case of children vertically infected by HBeAg-positive mothers, the duration may be over three decades. However, in children that are horizontally infected, the immune-tolerance phase seems to be very short and is hardly recognizable (2). Chang et al. reported that the annual rate of spontaneous HBeAg seroconversion is less than 2% in younger children (<3 years) and 4–5% in older children (5,6). However, around 90% of children are HBeAg-positive by 10–15 years of age (7). In Taiwan, the most common genotype in children with chronic HBV infections is B (>70%) and HBeAg seroconversion is slower in children with genotype C (8). The proportion of children with CHB entering the early immune-clearance phase differs according to age. In Korea, where genotype C is predominant, this proportion, which was calculated by the Kaplan-Meier method, was 4.6%, 11.7%, and 39.7% in children <6, <12, and <18 years, respectively (9).
Medications approved for CHB patients (≥2 years)
The US Food and Drug Administration (FDA) has approved five medications for treating CHB for children: interferon (IFN)-alpha, lamivudine, adefovir, entecavir, and, recently, tenofovir (2). On March 20, 2014, the FDA approved expanding the patient population for treatment with entecavir to include pediatric CHB subjects 2 years and older. Tenofovir was recently approved for use for children over 12 years. Phase III trials using tenofovir for children 2 to 12 years are being conducted with a dose of 8 mg/kg (maximum dose 300 mg/day). Lamivudine was withdrawn from the first-line treatment group in adults because of very high resistance rates, but is still thought to be highly effective in children under 6 years in Korea where genotype C is predominant (10).
The antiviral effects of lamivudine, entecavir, and tenofovir on toddlers and preschoolers (<6 years)
The choice of antiviral medications with a low resistance profile and strong therapeutic potential is critical for first-line therapy in children and adults (11). Choe et al. (12) reported that long-term lamivudine treatment was more effective in achieving HBeAg seroconversion than IFN-alpha treatment, and this effect was also observed in toddlers and preschoolers. By the way, when entecavir is chosen as the first treatment option and viral resistance occurs due to poor patient compliance or false treatment indication, it is difficult to select a second-line treatment. However, if lamivudine is chosen as the first treatment option for younger children, even if the initial treatment fails, tenofovir can be considered as a rescue therapy (13,14). Until now, several factors such as a younger age at diagnosis, longer consolidation treatment period, lower HBV DNA levels at the end of treatment, and genotype B infection have been reported to be closely related with the elevated durability of lamivudine therapy (15-19). These factors could be applied to newly developed nucleos(t)ide analogues (NAs), as well.
Therapeutic choices for children between 2 and 12 years with CHB have been limited. Recently, several studies about the efficacy and safety of entecavir in children and adolescents have been reported. According to a recently published study by Jonas et al., entecavir was superior to a placebo in achieving normalized ALT levels, HBV DNA suppression, and HBeAg seroconversion at 48 weeks. Similar to adults, the appearance of viral resistance seemed less common with entecavir than with which other medications; the cumulative probability of entecavir resistance was 2.6% at years 2 (20).
The relation between the efficacy of NA treatment and genotypes
Lamivudine treatment responses and genotypes
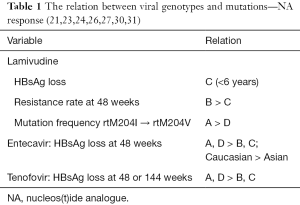
Pediatric patients have a good tolerance for lamivudine (21). Kim et al. showed that among patients treated with lamivudine, younger children (<7 years) achieved a higher rate of HBsAg clearance than older children in areas where genotype C was predominant. Among 49 children who achieved HBeAg seroconversion, 13/49 (26.5%) children lost HBsAg, even though they were all infected vertically (22).
Zöllner et al. (23) suggested that differences in viral activity were observed in vivo between genotype A and genotype D HBV with lamivudine resistance. Specifically, the HBV genotype appeared to influence the stability of codon rt204, and the resistance-associated mutations from rtM204I to rtM204V were significantly more common in genotype A carriers than in genotype D carriers.
A retrospective study reported that the development of lamivudine resistance was much higher in genotype B than genotype C within the initial 48 weeks of lamivudine therapy (24). Although the frequency of the HBV rtM204I mutation was higher in genotype B patients (67%) than in the mutation frequency related to genotype C patients, the difference was not statistically significant. This study suggested that practitioners needed to monitor changes in resistance in specific HBV genotypes while NAs were used for therapy.
Entecavir treatment responses and genotypes
Entecavir is highly effective in viral suppression in children and adolescents with CHB, but has a limitation in accomplishing complete remission.
In an adult study, entecavir was effective in sustained suppression of HBV DNA to a level of <300 copies/mL. The risk of antiviral drug resistance was minimal during the treatment period and virological rebound or genotypic resistance to entecavir was not observed (25). Another adult study showed the possibility of HBsAg loss with entecavir monotherapy. According to this retrospective analysis, all patients that achieved HBsAg loss by entecavir monotherapy maintained a low HBV DNA level (<300 copies/mL). Specifically, Caucasian patients and patients with HBV genotype A or D infections, had a significantly higher chance of losing HBsAg than Asian patients (26). However, the effect of HBV genotypes on HBsAg loss may be more apparent when studied according to ethnicity or the country of origin (or route of infection) (27).
A study conducted in Arab countries reported the results of treating eight HBeAg-positive children (mean age 4.8 years; range, 2.6–15 years) with HBV genotype D infections. This study concluded that 37.5% of children treated with entecavir achieved HBeAg loss and seroconversion without any side effects during the treatment period (28). Chu et al. (29) reported that both the lamivudine and adefovir combination therapy and entecavir monotherapy were superior to adefovir monotherapy in viral suppression in children and adolescents with lamivudine-resistant CHB with HBV genotype C infections, but the difference between the two groups was not significant. Currently, tenofovir is used for rescue treatment in lamivudine-resistant CHB.
Tenofovir treatment responses and genotypes
According to a study by van Bömmel et al. (13), HBeAg seroconversion was achieved in 20 of 85 HBeAg-positive patients (24%) after a median duration of 11 months of tenofovir disoproxil fumarate (TDF) treatment. HBsAg loss was observed in 4 patients (3%) with HBV genotype A infections after 25 months of TDF treatment. A recent study about TDF treatment response stated that among 158 HBeAg-positive patients, 5 patients lost HBsAg at 48 weeks of TDF monotherapy (30). The genotypes of these five patients were A (two patients) and D (three patients). Even though there are limitations in drawing a conclusion using data from a small study size, this study also showed the relation between specific HBV genotypes and HBsAg loss induced by NAs.
In addition, Heathcote et al. reported that in an adult study (31), HBeAg-positive patients treated with TDF who lost HBsAg included genotypes A (60%), D (35%), and F (5%) after 144 weeks of treatment. Especially in the genotype A group, among 46 HBeAg-positive patients, 12 patients lost HBsAg, and in the genotype D group, among 78 patients, 7 lost HBsAg. This result implied that the therapeutic effects of TDF differ among CHB patients infected with different genotypes. It is critical to check the genotypes of patients for better therapeutic results, especially when considering TDF as the first-line choice for CHB patients (Table 1).
Although there are no published studies about the efficacy of TDF on children with CHB, it seems that pediatricians should consider TDF as the main treatment regimen for patients with CHB.
CHB treatment according to specific genotypes
Patients with HBV genotype C usually experience a longer period of active HBV replication, which results in delayed HBeAg seroconversion. Therefore, they are at risk for more severe fibrosis, cirrhosis, and even HCC than genotype B patients (8,32,33). Spontaneous HBsAg seroclearance is more common in genotype A and genotype B patients compared with genotype C and genotype D patients (34,35).
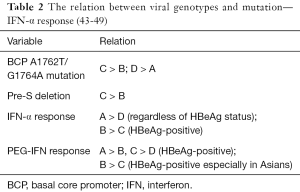
Several studies about the response rate to IFN therapy have revealed that the rates of HBeAg seroconversion are significantly higher in patients infected with genotype B than genotype C (36,37). A number of studies have also showed that the therapeutic effect of PEG-IFN-α-2b on HBsAg clearance is highest in genotype A patients (38,39).
Standard and PEG IFN-α was proven to be more effective on people with HBV genotypes A and B than those with genotypes C and D (40). Many studies reported that the therapeutic responses to IFN therapy were higher in genotype A than genotype D and higher in genotype B than genotype C (41).
According to a study by Sugiyama et al. that used both in vivo and in vitro experimental systems, genotype D had higher intracellular levels of HBV DNA and core protein than genotype A, and this may explain why genotype D is more resistant to IFN therapy than genotype A (42) (Table 2).
The relation between the response to NAs and HBV genotype have not been clarified (43). However, recently, a few studies suggested that each NA and genotype reveals differences in resistance and curability, respectively (13,23,24,26,30,31). If this were true, then it would be beneficial to select different first-line drugs for specific genotypes.
The distribution of specific genotypes is as follows: genotype A is common in sub-Saharan Africa, India, Western Africa, and Northern Europe; genotypes B and C are prevalent in Asia; genotype C chiefly exists in Southeast and Far East Asia; and genotype D is popular in Africa, India, the Mediterranean region, and Europe (50).
Monitoring after complete remission
It is important to prevent relapse after completing HBeAg seroconversion with lamivudine monotherapy by continuing treatment for more than at least 6 months after HBeAg seroconversion (51). However, Hong et al. (10) reported that lamivudine should be used until achieving complete remission and that it is needed for more than at least 12 months after HBeAg/anti-HBe seroconversion to get better therapeutic results, even in young children. Authors suggest that NAs should be continued for 2 years after complete remission if it takes a very long time to achieve HBeAg seroconversion or if low HBV DNA levels persist after complete remission.
Prevention of treatment failure
First, being in the immune-tolerance phase should not be considered as a treatment indication. The initial ALT level must be higher than 2× ULN to avoid resistance (10).
Second, if a high ALT level persists in a patient, then other hepatic problems should also be considered including reactive hepatitis in children with other infections such as pneumonia and acute pyelonephritis. For an obese child with CHB, it may not be possible to identify whether the cause of ALT elevation is due to an active HBV infection or nonalcoholic fatty liver disease; therefore, a liver biopsy should be performed to determine whether to treat the patient or not if the patient failed to reduce body weight.
Third, poor compliance is another cause of treatment failure. Practitioners should educate CHB patients about the importance of daily administration of medicines without omission.
Fourth, it is also important not to stop medication too early after achieving complete remission. An additional period of treatment must be applied for 1 or 2 years after HBeAg seroconversion to prevent relapse.
Conclusions
Newly developed NAs are potent in children with CHB. Physicians should update their information about the current treatment guidelines to improve therapeutic efficacy. Pretreatment determination of the specific genotype might be helpful in the preparation of treatment strategy for children with CHB.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jonas MM, Block JM, Haber BA, et al. Treatment of children with chronic hepatitis B virus infection in the United States: patient selection and therapeutic options. Hepatology 2010;52:2192-205. [Crossref] [PubMed]
- Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1-98. [Crossref] [PubMed]
- European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol 2012;57:167-85. [Crossref] [PubMed]
- Kao JH, Chen DS. Critical analysis of the immune tolerance phase of chronic HBV infection: natural history and diagnosis. Current Hepatitis Reports 2008;7:5-11. [Crossref]
- Chang MH. Hepatitis B virus infection. Semin Fetal Neonatal Med 2007;12:160-7. [Crossref] [PubMed]
- Chang MH, Hsu HY, Hsu HC, et al. The significance of spontaneous hepatitis B e antigen seroconversion in childhood: with special emphasis on the clearance of hepatitis B e antigen before 3 years of age. Hepatology 1995;22:1387-92. [PubMed]
- Chu CM, Liaw YF. Chronic hepatitis B virus infection acquired in childhood: special emphasis on prognostic and therapeutic implication of delayed HBeAg seroconversion. J Viral Hepat 2007;14:147-52. [Crossref] [PubMed]
- Ni YH, Chang MH, Wang KJ, et al. Clinical relevance of hepatitis B virus genotype in children with chronic infection and hepatocellular carcinoma. Gastroenterology 2004;127:1733-8. [PubMed]
- Hong SJ, Park HJ, Chu MA, et al. The Rate of Conversion from Immune-tolerant Phase to Early Immune-clearance Phase in Children with Chronic Hepatitis B Virus Infection. Pediatr Gastroenterol Hepatol Nutr 2014;17:41-6. [Crossref] [PubMed]
- Hong SJ, Kim YH, Choe BH, et al. Current role of Lamivudine regarding therapeutic response and resistance in children with chronic hepatitis B. Pediatr Gastroenterol Hepatol Nutr 2013;16:80-8. [Crossref] [PubMed]
- Kasırga E. Lamivudine resistance in children with chronic hepatitis B. World J Hepatol 2015;7:896-902. [Crossref] [PubMed]
- Choe BH, Lee JH, Jang YC, et al. Long-term therapeutic efficacy of lamivudine compared with interferon-alpha in children with chronic hepatitis B: the younger the better. J Pediatr Gastroenterol Nutr 2007;44:92-8. [Crossref] [PubMed]
- van Bömmel F, de Man RA, Wedemeyer H, et al. Long-term efficacy of tenofovir monotherapy for hepatitis B virus-monoinfected patients after failure of nucleoside/nucleotide analogues. Hepatology 2010;51:73-80. [Crossref] [PubMed]
- Lada O, Benhamou Y, Cahour A, et al. In vitro susceptibility of lamivudine-resistant hepatitis B virus to adefovir and tenofovir. Antivir Ther 2004;9:353-63. [PubMed]
- Song BC, Suh DJ, Lee HC, et al. Hepatitis B e antigen seroconversion after lamivudine therapy is not durable in patients with chronic hepatitis B in Korea. Hepatology 2000;32:803-6. [Crossref] [PubMed]
- Lee KM, Cho SW, Kim SW, et al. Effect of virological response on post-treatment durability of lamivudine-induced HBeAg seroconversion. J Viral Hepat 2002;9:208-12. [Crossref] [PubMed]
- Ryu SH, Chung YH, Choi MH, et al. Long-term additional lamivudine therapy enhances durability of lamivudine-induced HBeAg loss: a prospective study. J Hepatol 2003;39:614-9. [Crossref] [PubMed]
- Lee HC, Suh DJ, Ryu SH, et al. Quantitative polymerase chain reaction assay for serum hepatitis B virus DNA as a predictive factor for post-treatment relapse after lamivudine induced hepatitis B e antigen loss or seroconversion. Gut 2003;52:1779-83. [Crossref] [PubMed]
- Chien RN, Yeh CT, Tsai SL, et al. Determinants for sustained HBeAg response to lamivudine therapy. Hepatology 2003;38:1267-73. [Crossref] [PubMed]
- Jonas MM, Chang MH, Sokal E, et al. Randomized, controlled trial of entecavir versus placebo in children with hepatitis B envelope antigen-positive chronic hepatitis B. Hepatology 2016;63:377-87. [Crossref] [PubMed]
- Chan HL, Chen YC, Gane EJ, et al. Randomized clinical trial: efficacy and safety of telbivudine and lamivudine in treatment-naïve patients with HBV-related decompensated cirrhosis. J Viral Hepat 2012;19:732-43. [Crossref] [PubMed]
- Kim JM, Choe BH, Chu MA, et al. Comparison of lamivudine-induced HBsAg loss rate according to age in children with chronic hepatitis B. Korean J Hepatol 2009;15:168-78. [Crossref] [PubMed]
- Zöllner B, Petersen J, Puchhammer-Stöckl E, et al. Viral features of lamivudine resistant hepatitis B genotypes A and D. Hepatology 2004;39:42-50. [Crossref] [PubMed]
- Hsieh TH, Tseng TC, Liu CJ, et al. Hepatitis B virus genotype B has an earlier emergence of lamivudine resistance than genotype C. Antivir Ther 2009;14:1157-63. [Crossref] [PubMed]
- Chang TT, Liaw YF, Wu SS, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology 2010;52:886-93. [Crossref] [PubMed]
- Gish RG, Chang TT, Lai CL, et al. Loss of HBsAg antigen during treatment with entecavir or lamivudine in nucleoside-naïve HBeAg-positive patients with chronic hepatitis B. J Viral Hepat 2010;17:16-22. [Crossref] [PubMed]
- Gish RG, Chang TT, Lai CL, et al. Quantitative hepatitis B surface antigen analysis in hepatitis B e antigen-positive nucleoside-naive patients treated with entecavir. Antivir Ther 2013;18:691-8. [Crossref] [PubMed]
- Saadah OI, Sindi HH, Bin-Talib Y, et al. Entecavir treatment of children 2-16 years of age with chronic hepatitis B infection. Arab J Gastroenterol 2012;13:41-4. [Crossref] [PubMed]
- Chu M, Cho SM, Choe BH, et al. Virologic responses to add-on adefovir dipivoxil treatment versus entecavir monotherapy in children with lamivudine-resistant chronic hepatitis B. J Pediatr Gastroenterol Nutr 2012;55:648-52. [Crossref] [PubMed]
- Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med 2008;359:2442-55. [Crossref] [PubMed]
- Heathcote EJ, Marcellin P, Buti M, et al. Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology 2011;140:132-43. [Crossref] [PubMed]
- Chu CJ, Hussain M, Lok AS. Hepatitis B virus genotype B is associated with earlier HBeAg seroconversion compared with hepatitis B virus genotype C. Gastroenterology 2002;122:1756-62. [Crossref] [PubMed]
- Watanabe K, Takahashi T, Takahashi S, et al. Comparative study of genotype B and C hepatitis B virus-induced chronic hepatitis in relation to the basic core promoter and precore mutations. J Gastroenterol Hepatol 2005;20:441-9. [Crossref] [PubMed]
- Sánchez-Tapias JM, Costa J, Mas A, et al. Influence of hepatitis B virus genotype on the long-term outcome of chronic hepatitis B in western patients. Gastroenterology 2002;123:1848-56. [Crossref] [PubMed]
- Yuen MF, Wong DK, Sablon E, et al. HBsAg seroclearance in chronic hepatitis B in the Chinese: virological, histological, and clinical aspects. Hepatology 2004;39:1694-701. [Crossref] [PubMed]
- Kao JH, Wu NH, Chen PJ, et al. Hepatitis B genotypes and the response to interferon therapy. J Hepatol 2000;33:998-1002. [Crossref] [PubMed]
- Wai CT, Chu CJ, Hussain M, et al. HBV genotype B is associated with better response to interferon therapy in HBeAg(+) chronic hepatitis than genotype C. Hepatology 2002;36:1425-30. [PubMed]
- Buster EH, Flink HJ, Cakaloglu Y, et al. Sustained HBeAg and HBsAg loss after long-term follow-up of HBeAg-positive patients treated with peginterferon alpha-2b. Gastroenterology 2008;135:459-67. [Crossref] [PubMed]
- Flink HJ, van Zonneveld M, Hansen BE, et al. Treatment with Peg-interferon alpha-2b for HBeAg-positive chronic hepatitis B: HBsAg loss is associated with HBV genotype. Am J Gastroenterol 2006;101:297-303. [Crossref] [PubMed]
- Janssen HL, van Zonneveld M, Senturk H, et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet 2005;365:123-9. [Crossref] [PubMed]
- Liu CJ, Kao JH, Chen DS. Therapeutic implications of hepatitis B virus genotypes. Liver Int 2005;25:1097-107. [Crossref] [PubMed]
- Sugiyama M, Tanaka Y, Kato T, et al. Influence of hepatitis B virus genotypes on the intra- and extracellular expression of viral DNA and antigens. Hepatology 2006;44:915-24. [Crossref] [PubMed]
- Wiegand J, Hasenclever D, Tillmann HL. Should treatment of hepatitis B depend on hepatitis B virus genotypes? A hypothesis generated from an explorative analysis of published evidence. Antivir Ther 2008;13:211-20. [PubMed]
- Yu MW, Yeh SH, Chen PJ, et al. Hepatitis B virus genotype and DNA level and hepatocellular carcinoma: a prospective study in men. J Natl Cancer Inst 2005;97:265-72. [Crossref] [PubMed]
- Sharma S, Sharma B, Singla B, et al. Clinical significance of genotypes and precore/basal core promoter mutations in HBV related chronic liver disease patients in North India. Dig Dis Sci 2010;55:794-802. [Crossref] [PubMed]
- Liu S, Zhang H, Gu C, et al. Associations between hepatitis B virus mutations and the risk of hepatocellular carcinoma: a meta-analysis. J Natl Cancer Inst 2009;101:1066-82. [Crossref] [PubMed]
- Buster EH, Hansen BE, Lau GK, et al. Factors that predict response of patients with hepatitis B e antigen-positive chronic hepatitis B to peginterferon-alfa. Gastroenterology 2009;137:2002-9. [Crossref] [PubMed]
- Tseng TC, Yu ML, Liu CJ, et al. Effect of host and viral factors on hepatitis B e antigen-positive chronic hepatitis B patients receiving pegylated interferon-α-2a therapy. Antivir Ther 2011;16:629-37. [Crossref] [PubMed]
- Kao JH. HBeAg-positive chronic hepatitis B: why do I treat my patients with pegylated interferon? Liver Int 2014;34 Suppl 1:112-9. [Crossref] [PubMed]
- Liu CJ, Kao JH. Global perspective on the natural history of chronic hepatitis B: role of hepatitis B virus genotypes A to J. Semin Liver Dis 2013;33:97-102. [Crossref] [PubMed]
- Broderick A, Jonas MM. Management of hepatitis B in children. Clin Liver Dis 2004;8:387-401. [Crossref] [PubMed]