Diagnosis of hepatitis B
Introduction
Hepatitis B virus (HBV) belongs to the Hepadnaviridae family. It shows a diameter of 30–42 nm and consists of outer lipid envelope containing hepatitis B surface antigen (HBsAg) and an icosahedral capsid core composed of protein (1). Viral capsid bears viral genome and DNA polymerase that has reverse transcriptase activity. HBV genome comprise a circular, partly double-stranded DNA and has four open reading frames overlapped: (I) S that encodes for surface proteins (HBsAg); (II) pre-C/C for hepatitis B e antigen (HBeAg) and core protein (HBcAg); (III) P for polymerase including reverse transcriptase; (IV) X that encodes for a transcriptional transactivator factor (HBxAg) (2). The covalently closed circular DNA (cccDNA) is the transcriptional template of HBV and stays inside the hepatocyte nucleus as a mini-chromosome (3). Reverse transcriptase involved in the replication of HBV is error susceptible, thus the mutation rate is high, similarly observed in retroviruses and RNA viruses (4,5).
HBV infection is responsible for the most of chronic liver diseases worldwide and is transmitted through parenteral, sexual and vertical route. About 240 million people are chronically infected by HBV, so have the risk of developing of liver cirrhosis and hepatocellular carcinoma (HCC). According to HBsAg prevalence, HBV endemicity is divided into three categories; high, intermediate, low. China, South East Asia, Indonesia, and sub-Saharan Africa are regarded as highly endemic areas because chronic HBV infection is reported in more than 8% of the population (6). Intermediate areas show chronic HBV infection rate between 2% and 7% of population, and include South America, South West Asia, Eastern and Southern Europe. Developed countries, such as North America and Western Europe are grouped as low endemic regions; in these areas, HBV prevalence rates range from 0.5% to 2%.
Along with active anti-HBV vaccination, one significant method to diminish the burden of this disease is the diagnosis of acute, chronic and occult HBV infection. In this article, the serological and molecular diagnosis of HBV will be reviewed.
Serological markers for HBV infection
Serological markers for HBV infection consist of HBsAg, anti-HBs, HBeAg, anti-HBe, and anti-HBc IgM and IgG. The identification of serological markers allows: to identify patients with HBV infection; to elucidate the natural course of chronic hepatitis B (CHB); to assess the clinical phases of infection; and to monitor antiviral therapy (7).
HBsAg is the serological hallmark of HBV infection. After an acute exposure to HBV, HBsAg appears in serum within 1 to 10 weeks. Persistence of this marker for more than 6 months implies chronic HBV infection (8). Several studies have reported the association between transcription activity of cccDNA in the liver and serum HBsAg levels (9-11). Differences in the serum HBsAg levels during the different phases of infection indicate the distribution of cccDNA during the respective phases of the disease. The serum HBsAg titers are higher in patients with HBeAg-positive CHB than in HBeAg-negative CHB (10-12). Monitoring of quantitative HBsAg levels predicts treatment response to interferon and disease progression in HBeAg-negative CHB patients with normal serum alanine aminotransferase levels (13,14).
Anti-HBs is known as a neutralizing antibody, and confers long-term immunity (15). In patients with acquired immunity through vaccination, anti-HBs is the only serological marker detected in serum. In the past HBV infection, it is present in concurrence with anti-HBc IgG. Occasionally, the simultaneous appearance of HBsAg and anti-HBs has been reported in patients with HBsAg positive (16). In most cases, anti-HBs antibodies are unable to neutralize the circulating viruses, thus these patients are regarded as carriers of HBV.
In the past, HBeAg and anti-HBe had been used to know infectivity and viral replication, but their use for this purpose has mostly been replaced by HBV DNA assay. HBeAg to anti-HBe seroconversion is related to the remission of hepatic disease (17), however, active viral replication is sustained in some patients with HBe seroconversion due to mutations in the pre-core and core region that inhibit or decrease the production of HBeAg (8).
HBcAg is an intracellular presence in infected hepatocyte, thus it is not identified in the serum. During acute infection, anti-HBc IgM and IgG emerges 1–2 weeks after the presence of HBsAg along with raised serum aminotransferase and symptoms. After 6 months of acute infection, anti-HBc IgM wears off. Anti-HBc IgG continues to detect in both patients with resolved HBV infection and CHB. Some HBsAg-negative individuals are positive for anti-HBc IgG without anti-HBs, in this situation, it should be considered isolated anti-HBc positive. It can be seen in three conditions. First, it can be predominantly seen as IgM class during the window period of acute phase. Second, after acute infection had ended, anti-HBs has decreased below the cutoff level of detection. Third, after several years of chronic HBV infection, HBsAg has diminished to undetectable levels. If the result of serological markers shows isolated anti-HBc positive, anti-HBc IgM should be checked in order to assess the possibility of recent HBV exposure. HBV DNA assays should be tested in chronic liver disease patients to find out occult HBV infection characterized by existence of detectable HBV DNA without serum HBsAg (18).
Molecular methods for HBV infection
HBV DNA is a direct measurement of the viral load, which reveals the replication activity of the virus. It is detectable at the early stage of infection (1 month after HBV infection) and increases up to peak level (more than 108 copies/mL) approximately 3 months after the exposure to HBV and then gradually diminishes in chronic infection or disappears at the recovery from HBV infection.
As the prevalence of serologically negative HBV infection (HBeAg-negative CHB and occult HBV infection) has increased, HBV-DNA detection has obtained more awareness in clinical medicine (19). The detection of HBV DNA is a reliable marker of replication activity, and higher titers of HBV DNA are related to the more rapid disease progression and higher incidence of HCC (20). Furthermore, HBV DNA testing is useful in routine clinical setting to determine patients who need antiviral therapy and monitor them for suitable treatment (21).
There are two principles of techniques to identify and quantify HBV DNA: signal amplification such as hybrid capture and branched DNA technology; target amplification such as polymerase chain reaction (PCR) (19,22). Real-time PCR can detect wide dynamic range of viral load (lower range, 10–15 IU/mL; upper range, 107–108 IU/mL). For this reason, it has come to be the standard method to detect and quantify HBV DNA in clinical setting. Furthermore, it can be fully automated and does not generate carry-over contamination (23). Table 1 displays the comparison of assays for quantitative measurement of HBV DNA.

Full table
HBV genotyping
HBV has a high genetic heterogeneity because it reproduces via a reverse transcriptase that has insufficient proofreading capability. According to the sequence divergence, HBV can be divided into ten genotypes, labelled A–J: they have distinct geographic distribution (24). Genotype B and C are restricted to Oceania and Asia, whereas genotype A and D are omnipresent but most common in Africa and Europe (25). Genotype I is unusual and can be observed in Vietnam, Laos, India and China, while genotype J has been reported in Japan and Ryukyu (26,27). Other genotypes such as E, F, G, and H are also occasionally found in Asia.
Evidences increasingly suggest that the HBV genotyping is significant to predict HBV disease progression and determine appropriate antiviral therapy. Acute infection with genotypes A and D leads to higher rate of chronicity than genotypes B and C (28-30). Genotype C generally is considered as a risk factor for perinatal infection (31) and related to severe liver disease, including cirrhosis and HCC (32-34). In the interferon therapy, patients with genotypes A and B have better treatment response than genotypes C and D (35). Recent studies reported that patients infected with genotype B or C had a lower opportunity to gain serological response to tenofovir (36,37).
HBV genotyping can be confirmed using diverse methods: reverse hybridization, genotype-specific PCR assays, real-time PCR, restriction fragment-length polymorphism, sequence analysis, microarray (DNAChip) and fluorescence polarization assay (38). The characteristics of variable HBV genotyping methods are presented on Table 2.
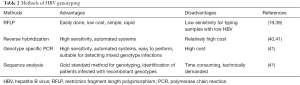
Full table
Diagnosis of hepatitis B infection
Acute hepatitis B is a clinical diagnosis identified by the detection of HBsAg, symptoms, high serum aminotransferases. Usually anti-HBc IgM can be detected and HBV DNA is present. HBeAg can also be identified in most acute phase of infections, but has little clinical importance. The diagnosis of chronic infection is based on the persistence of HBsAg for more than 6 months. Patients with chronic HBV infection are commonly diagnosed by laboratory means but not by clinical presentations. Past HBV infection is defined by the coexistence of anti-HBs and IgG anti-HBc.
Occult HBV infection is defined by persistence of low level of intrahepatic HBV DNA without detectable HBsAg (42,43). It is a serological situation defined by the presence of isolated anti-HBc with the absence of HBsAg and anti-HBs antibody (44,45). The detection of HBV DNA in the liver is the gold standard of diagnosis for occult HBV infection, since cccDNA remains in the hepatocytes and HBV DNA is occasionally identified in the liver but not in the serum. However, gaining hepatic HBV DNA is difficult in clinical setting since the procedure is invasive. Real-time PCR for serum HBV DNA detection have been shown with adequate sensitivity to identify occult HBV infection in many cases; thus, HBV DNA testing is widely used to diagnose occult HBV infection (43).
Occult HBV infection has some clinical importance. First, it can be transmitted via transfusion, solid organ transplantation including orthotopic liver transplantation (46,47), or hemodialysis (48,49). Second, reactivation of HBV infection may occur in patients receiving chemotherapy or immunocompromised state (50-52). Third, it may accelerate liver injury and lead to hepatic fibrosis in patients with chronic liver disease including chronic hepatitis C infection (53-55). Forth, it appears to be a risk factor for HCC by its carcinogenic effect and by leading to continuous hepatic inflammation and fibrosis (56-58).
Tests for occult HBV infection are considered in the following conditions: in patients with cryptogenic liver disease, especially when having anti-HBc in serum; in patients considering immunosuppression therapy or chemotherapy; and in solid organ transplantation donors, due to the possibilities for transmission (59).
Conclusions
In this article, we aimed to give informations about HBV serological and molecular diagnosis. First step of HBV diagnosis is achieved by using serological markers for detecting antigens and antibodies against this virus. In order to verify first step of diagnosis, to quantify viral load and to identify genotypes, qualitative or quantitative molecular tests are used. Diagnosis of HBV infection is an important tool to determine acute, chronic and occult cases of infection in order to establish preventive remedies and to initiate antiviral treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Takahashi T, Nakagawa S, Hashimoto T, et al. Large-scale isolation of Dane particles from plasma containing hepatitis B antigen and deomnstration of circular double-stranded DNA molecule extruding directly from their cores. J Immunol 1976;117:1392-7. [PubMed]
- Kramvis A. Genotypes and genetic variability of hepatitis B virus. Intervirology 2014;57:141-50. [Crossref] [PubMed]
- Locarnini S, Zoulim F. Molecular genetics of HBV infection. Antivir Ther 2010;15 Suppl 3:3-14. [Crossref] [PubMed]
- Ganem D, Schneider RJ. Hepadnaviridae: the viruses and their replication. Fields Virology 2001;2:2923-69.
- Simmonds P. Reconstructing the origins of human hepatitis viruses. Philos Trans R Soc Lond B Biol Sci 2001;356:1013-26. [Crossref] [PubMed]
- Ott JJ, Stevens GA, Groeger J, et al. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine 2012;30:2212-9. [Crossref] [PubMed]
- Control CfD, Prevention. Epidemiology and prevention of vaccine-preventable diseases. Washington DC: Public Health Foundation, 2011;12.
- Kao JH. Diagnosis of hepatitis B virus infection through serological and virological markers. Expert Rev Gastroenterol Hepatol 2008;2:553-62. [Crossref] [PubMed]
- Chan HL, Wong VW, Tse AM, et al. Serum hepatitis B surface antigen quantitation can reflect hepatitis B virus in the liver and predict treatment response. Clin Gastroenterol Hepatol 2007;5:1462-8. [Crossref] [PubMed]
- Nguyen T, Thompson AJ, Bowden S, et al. Hepatitis B surface antigen levels during the natural history of chronic hepatitis B: a perspective on Asia. J Hepatol 2010;52:508-13. [Crossref] [PubMed]
- Thompson AJ, Nguyen T, Iser D, et al. Serum hepatitis B surface antigen and hepatitis B e antigen titers: disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology 2010;51:1933-44. [Crossref] [PubMed]
- Jaroszewicz J, Calle Serrano B, Wursthorn K, et al. Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)-infection: a European perspective. J Hepatol 2010;52:514-22. [Crossref] [PubMed]
- Chan HL, Thompson A, Martinot-Peignoux M, et al. Hepatitis B surface antigen quantification: why and how to use it in 2011 - a core group report. J Hepatol 2011;55:1121-31. [Crossref] [PubMed]
- Martinot-Peignoux M, Carvalho-Filho R, Lapalus M, et al. Hepatitis B surface antigen serum level is associated with fibrosis severity in treatment-naïve, e antigen-positive patients. J Hepatol 2013;58:1089-95. [Crossref] [PubMed]
- Weber B. Recent developments in the diagnosis and monitoring of HBV infection and role of the genetic variability of the S gene. Expert Rev Mol Diagn 2005;5:75-91. [Crossref] [PubMed]
- Tsang TK, Blei AT, O'Reilly DJ, et al. Clinical significance of concurrent hepatitis B surface antigen and antibody positivity. Dig Dis Sci 1986;31:620-4. [Crossref] [PubMed]
- Dény P, Zoulim F. Hepatitis B virus: from diagnosis to treatment. Pathol Biol (Paris) 2010;58:245-53. [Crossref] [PubMed]
- Raimondo G, Pollicino T, Cacciola I, et al. Occult hepatitis B virus infection. J Hepatol 2007;46:160-70. [Crossref] [PubMed]
- Datta S, Chatterjee S, Veer V. Recent advances in molecular diagnostics of hepatitis B virus. World J Gastroenterol 2014;20:14615-25. [Crossref] [PubMed]
- Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006;295:65-73. [Crossref] [PubMed]
- Chevaliez S, Pawlotsky JM. Diagnosis and management of chronic viral hepatitis: antigens, antibodies and viral genomes. Best Pract Res Clin Gastroenterol 2008;22:1031-48. [Crossref] [PubMed]
- Caliendo AM, Valsamakis A, Bremer JW, et al. Multilaboratory evaluation of real-time PCR tests for hepatitis B virus DNA quantification. J Clin Microbiol 2011;49:2854-8. [Crossref] [PubMed]
- Bustin SA, Benes V, Nolan T, et al. Quantitative real-time RT-PCR--a perspective. J Mol Endocrinol 2005;34:597-601. [Crossref] [PubMed]
- Lin CL, Kao JH. The clinical implications of hepatitis B virus genotype: Recent advances. J Gastroenterol Hepatol 2011;26 Suppl 1:123-30. [Crossref] [PubMed]
- Zehender G, Ebranati E, Gabanelli E, et al. Enigmatic origin of hepatitis B virus: an ancient travelling companion or a recent encounter? World J Gastroenterol 2014;20:7622-34. [Crossref] [PubMed]
- Li GJ, Hue S, Harrison TJ, et al. Hepatitis B virus candidate subgenotype I1 varies in distribution throughout Guangxi, China and may have originated in Long An county, Guangxi. J Med Virol 2013;85:799-807. [Crossref] [PubMed]
- Shi YH. Correlation between hepatitis B virus genotypes and clinical outcomes. Jpn J Infect Dis 2012;65:476-82. [Crossref] [PubMed]
- Suzuki Y, Kobayashi M, Ikeda K, et al. Persistence of acute infection with hepatitis B virus genotype A and treatment in Japan. J Med Virol 2005;76:33-9. [Crossref] [PubMed]
- Kobayashi M, Suzuki F, Arase Y, et al. Infection with hepatitis B virus genotype A in Tokyo, Japan during 1976 through 2001. J Gastroenterol 2004;39:844-50. [Crossref] [PubMed]
- Wai CT, Fontana RJ, Polson J, et al. Clinical outcome and virological characteristics of hepatitis B-related acute liver failure in the United States. J Viral Hepat 2005;12:192-8. [Crossref] [PubMed]
- Ding Y, Sheng Q, Ma L, et al. Chronic HBV infection among pregnant women and their infants in Shenyang, China. Virol J 2013;10:17. [Crossref] [PubMed]
- Lee MH, Yang HI, Liu J, et al. Prediction models of long-term cirrhosis and hepatocellular carcinoma risk in chronic hepatitis B patients: risk scores integrating host and virus profiles. Hepatology 2013;58:546-54. [Crossref] [PubMed]
- Kim DW, Lee SA, Hwang ES, et al. Naturally occurring precore/core region mutations of hepatitis B virus genotype C related to hepatocellular carcinoma. PLoS One 2012;7:e47372. [Crossref] [PubMed]
- Lee SA, Kim K, Kim H, et al. Nucleotide change of codon 182 in the surface gene of hepatitis B virus genotype C leading to truncated surface protein is associated with progression of liver diseases. J Hepatol 2012;56:63-9. [Crossref] [PubMed]
- Kao JH, Wu NH, Chen PJ, et al. Hepatitis B genotypes and the response to interferon therapy. J Hepatol 2000;33:998-1002. [Crossref] [PubMed]
- Marcellin P, Buti M, Krastev Z, et al. Kinetics of hepatitis B surface antigen loss in patients with HBeAg-positive chronic hepatitis B treated with tenofovir disoproxil fumarate. J Hepatol 2014;61:1228-37. [Crossref] [PubMed]
- Chan HL, Chan CK, Hui AJ, et al. Effects of tenofovir disoproxil fumarate in hepatitis B e antigen-positive patients with normal levels of alanine aminotransferase and high levels of hepatitis B virus DNA. Gastroenterology 2014;146:1240-8. [Crossref] [PubMed]
- Pourkarim MR, Amini-Bavil-Olyaee S, Kurbanov F, et al. Molecular identification of hepatitis B virus genotypes/subgenotypes: revised classification hurdles and updated resolutions. World J Gastroenterol 2014;20:7152-68. [Crossref] [PubMed]
- Amini-Bavil-Olyaee S, Tacke F, Alavian SM. HBV Subgenotypes D1, D2, D-del! Are 'Old' Genotyping Methods Interpreted Correctly? Hepat Mon 2013;13:e13048. [Crossref] [PubMed]
- Osiowy C, Giles E. Evaluation of the INNO-LiPA HBV genotyping assay for determination of hepatitis B virus genotype. J Clin Microbiol 2003;41:5473-7. [Crossref] [PubMed]
- Ali MM, Hasan F, Ahmad S, et al. Comparative evaluation of INNO-LiPA HBV assay, direct DNA sequencing and subtractive PCR-RFLP for genotyping of clinical HBV isolates. Virol J 2010;7:111. [Crossref] [PubMed]
- Hollinger FB, Sood G. Occult hepatitis B virus infection: a covert operation. J Viral Hepat 2010;17:1-15. [Crossref] [PubMed]
- Raimondo G, Allain JP, Brunetto MR, et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol 2008;49:652-7. [Crossref] [PubMed]
- Grob P, Jilg W, Bornhak H, et al. Serological pattern "anti-HBc alone": report on a workshop. J Med Virol 2000;62:450-5. [Crossref] [PubMed]
- Weber B, Melchior W, Gehrke R, et al. Hepatitis B virus markers in anti-HBc only positive individuals. J Med Virol 2001;64:312-9. [Crossref] [PubMed]
- Raimondo G, Caccamo G, Filomia R, et al. Occult HBV infection. Semin Immunopathol 2013;35:39-52. [Crossref] [PubMed]
- Mahboobi N, Tabatabaei SV, Blum HE, et al. Renal grafts from anti-hepatitis B core-positive donors: a quantitative review of the literature. Transpl Infect Dis 2012;14:445-51. [Crossref] [PubMed]
- Minuk GY, Sun DF, Greenberg R, et al. Occult hepatitis B virus infection in a North American adult hemodialysis patient population. Hepatology 2004;40:1072-7. [Crossref] [PubMed]
- Yoo JH, Hwang SG, Yang DH, et al. Prevalence of occult hepatitis B virus infection in hemodialysis patients. Korean J Gastroenterol 2013;61:209-14. [Crossref] [PubMed]
- Kusumoto S, Tanaka Y, Mizokami M, et al. Reactivation of hepatitis B virus following systemic chemotherapy for malignant lymphoma. Int J Hematol 2009;90:13-23. [Crossref] [PubMed]
- Onozawa M, Hashino S, Izumiyama K, et al. Progressive disappearance of anti-hepatitis B surface antigen antibody and reverse seroconversion after allogeneic hematopoietic stem cell transplantation in patients with previous hepatitis B virus infection. Transplantation 2005;79:616-9. [Crossref] [PubMed]
- Yeo W, Johnson PJ. Diagnosis, prevention and management of hepatitis B virus reactivation during anticancer therapy. Hepatology 2006;43:209-20. [Crossref] [PubMed]
- Squadrito G, Cacciola I, Alibrandi A, et al. Impact of occult hepatitis B virus infection on the outcome of chronic hepatitis C. J Hepatol 2013;59:696-700. [Crossref] [PubMed]
- Kannangai R, Vivekanandan P, Netski D, et al. Liver enzyme flares and occult hepatitis B in persons with chronic hepatitis C infection. J Clin Virol 2007;39:101-5. [Crossref] [PubMed]
- Covolo L, Pollicino T, Raimondo G, et al. Occult hepatitis B virus and the risk for chronic liver disease: a meta-analysis. Dig Liver Dis 2013;45:238-44. [Crossref] [PubMed]
- Squadrito G, Pollicino T, Cacciola I, et al. Occult hepatitis B virus infection is associated with the development of hepatocellular carcinoma in chronic hepatitis C patients. Cancer 2006;106:1326-30. [Crossref] [PubMed]
- Obika M, Shinji T, Fujioka S, et al. Hepatitis B virus DNA in liver tissue and risk for hepatocarcinogenesis in patients with hepatitis C virus-related chronic liver disease. A prospective study. Intervirology 2008;51:59-68. [Crossref] [PubMed]
- Shi Y, Wu YH, Wu W, et al. Association between occult hepatitis B infection and the risk of hepatocellular carcinoma: a meta-analysis. Liver Int 2012;32:231-40. [Crossref] [PubMed]
- Valsamakis A. Molecular testing in the diagnosis and management of chronic hepatitis B. Clin Microbiol Rev 2007;20:426-39. table of contents. [Crossref] [PubMed]


