Association of insulin treatment versus oral hypoglycaemic agents with diabetic retinopathy and its severity in type 2 diabetes patients in Cameroon, sub-Saharan Africa
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disease leading to multi-systemic complications if left untreated. As its global prevalence increases year by year, it is a significant source of morbidity and mortality worldwide (1). This is particularly true for countries of sub-Saharan Africa such as Cameroon where prevalence and incidence rates grow faster than anywhere else, fueling epidemic proportions (2). The huge burden related to T2DM results from both macro and microvascular complications. The commonest microvascular complication of T2DM develops in eyes and is termed ‘diabetic retinopathy’ (DR) (1).
DR is a disease characterized by microvascular alterations progressively leading to retinal ischemia, retinal permeability, retinal neovascularization and macular edema (3,4). With regards to fundus photographies, the international clinical disease severity scale for DR has classified DR into five stages. The first is “no apparent retinopathy”. The second stage is “mild non-proliferative retinopathy” (NPDR) characterized by the presence of few microaneurysms. The third stage is “moderate NPDR” which is characterized by the presence of microaneurysms, intraretinal hemorrhages, or veinous beading but reduced compared with “severe NPDR” which is the fourth stage. “Severe NPDR” is characterized by no sign of proliferative diabetic retinopathy” (PDR) but any of the following: more than 20 intraretinal hemorrhages in each of four quadrants, definite veinous beading in two or more quadrants, prominent intraretinal microvascular anomalies in one or more quadrants. The fifth and last stage is “PDR” which is characterized by neovascularization of the disc, retina, iris, and angle; and by vitreous hemorrhage or tractional retinal detachment. Diabetic macular edema (DME) is commonly seen in DR patients. If DME is present, it can be further classified as mild, moderate, and severe depending on the distance of the exudates and thickening from the center of the fovea (4). DR is the leading cause of visual impairment and blindness in the populations aged 20–74 years as well as in diabetes patients worldwide. It may affect up to 60% of T2DM patients (5-9). Different risk and progression factors for DR have been documented; non-modifiable factors including genetic predisposition, ethnicity, gender, age, duration of diabetes, and modifiable factors including blood glucose, blood pressure, serum lipids and smoking (1).
Insulin is the mainstay in the treatment of diabetes mellitus including T2DM as a consequence of the progressive loss of pancreatic beta cell function (1). Recent insights from prospective studies carried out in developed countries and developing populations from Latin America and Asia have clearly demonstrated an incremented risk of DR with regard to insulin treatment (1,2,10,11). Of particular relevance, data regarding this topic are limited in sub-Saharan African populations who are however disproportionately affected by T2DM. In order to investigate the association between insulin treatment and both DR and its severity in a population from sub-Saharan Africa, we conducted this cross-sectional study.
Methods
Study design and setting
This study is reported with respect to the checklist recommended by Standard for Reporting Observational studies (STROBE) statement (12). It was a cross-sectional comparative study conducted at the Ophthalmology Department of the Douala General Hospital (DGH) between October 2004 and October 2006. The DGH is a tertiary health care hospital located in the Littoral Region of Cameroon, and was the only hospital setting that could perform retinal angiography and laser photocoagulation in the central African sub-region during the study period.
Patients’ characteristics
All T2DM patients aged ≥35 years, screened for DR at the Ophthalmology Department of the DGH and whose files had complete demographic and clinical data as well as information on eye examination were included and subsequently divided into two groups according to their respective treatments, insulin or oral hypoglycemic agent (OHA). Their selection in the two groups was additionally based on gender, age, sex, duration of diabetes, past history of hypertension, alcohol misuse, and current tobacco smoking. Eye examination comprised visual acuity testing, tonometry, and retinal exploration. Evaluation of the retina was made by Slit-Lamp biomicroscopy of the posterior pole using contact lens after pupil dilation with Tropicamide 0.5% and phenylephrine HCL 10% eyedrops; and retinal angiography after intravenous injection of 10% fluorescein. Retinal angiography findings were presented according to the shortened international version of the American Academy of Ophthalmology (13).
Sample size
We have previously reported a point prevalence of DR at 40.3% in the same setting (8). Assuming that this prevalence is 45% during the study period, and considering that the prevalence of T2DM patients under treatment with OHA is as 9 times as increased compared with that of T2DM patients treated with insulin (extrapolation from the ratio of proportion of type 2 to type 1 diabetes patients), with an 80% power to detect significant difference and an alpha error of 5%, the minimal sample size needed for this study was 66 subjects (60 cases of T2DM OHA treated patients, and 6 insulin treated patients).
Statistical analysis
Data were initially collected on questionnaires and subsequently entered in Excel software. All analyses were performed in SPSS for windows version 16 and Epi-Info for windows version 6 softwares. Whilst continuous variables are expressed as means with standard deviations (SDs), categorical variables are expressed as frequencies with their 95% confidence intervals (CIs). Means of continuous variables were compared using the analysis of variance (ANOVA) test while frequencies were compared using the chi square test (or the Fischer’s exact test when appropriate). A P value <0.05 was considered statistically significant for any difference. Neither sensitivity nor missing data analysis was carried out.
Results
The medical files of 134 T2DM patients were finally included in this analysis. Their ages ranged from 44 to 84 years, with a mean age of 59.3 (SD 7.9) years. The duration of diabetes ranged from 6 to 25 years, with a mean of 12.2 (SD 4.7) years. Demographic and clinical characteristics of the respective study groups are summarised in Table 1. There were significantly more OHA treated patients than insulin treated patients (82.8% vs. 17.2%, P<0.001). As expected, both the OHA and insulin groups were comparable by age, sex, duration of diabetes, past history of hypertension, alcohol misuse, and current tobacco smoking.
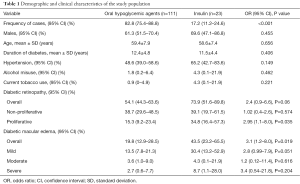
Full table
The frequency of DR was 54.1% among patients on OHA and 73.9% among those on insulin treatment, giving an overall frequency of 57.5%. The DR was almost significantly more frequent in T2DM patients under insulin regimen than in patients under OHA (73.9% vs. 54.1%; odds ratio (OR) 2.4; 95% CI, 0.9–6.6; P=0.06). Proliferative DR was significantly more observed in insulin treated patients than in OHA treated patients (34.8% vs. 15.3%; OR 2.95; 95% CI, 1.1–8; P=0.035). Irrespective of staging, the frequency of diabetic maculopathy was significantly higher in the insulin group than in the OHA group (43.5% vs. 19.8%; OR 3.1; 95% CI, 1.2–8; P=0.019).
Discussion
This study suggested that in sub-Saharan African T2DM patients, insulin therapy may be associated with DR, DR severity and DME when compared with OHA. Of note, the findings from this study were independent of patients’ age, sex, duration of diabetes, past history of hypertension, tobacco smoking and alcohol misuse given that they were comparable by these parameters known to increment the risk of incidental and progressive DR.
Cepeda-Nieto et al. found a significant association between insulin therapy and DR (P=0.0290) in a Mexican cross-sectional comparative study including T2DM patients. However, this was a negative association (OR 0.4026; 95% CI, 0.1793–0.9040) (14). Different methodological issues could account for this discrepancy. Nevertheless, our findings are supported by a growing body of evidence. Indeed, insulin use was an independent predisposing factor for DR (OR 32.71; 95% CI, 4.11–259.85) in the study by Javadi et al. (15), similarly as in the SN-DREAMS III cross-sectional study (OR 3.59; 95% CI, 1.41–9.14) (16). Consistent with those findings, a large retrospective cohort study including 85,214 T2DM patients identified insulin treatment as an independent risk factor for DR (hazard ratio 2.03; 95% CI, 1.89–2.18; P<0.001) (17). The pooled result of all the seven cohorts included in the meta-analysis by Zhao et al. also showed that insulin use is associated with incremented risk of DR (relative risk 2.3; 95% CI, 1.35–3.93) (1). Likewise, insulin treatment has been shown to be a risk factor for DME by previous studies. Aroca et al. observed that insulin use was a risk factor for focal and diffuse DME in a 4-year prospective study including 93 T2DM patients (18). This was recently demonstrated in the study by Zhang et al. (11). It is notable that these authors found an increased risk for DME associated with insulin use in a random-effect meta-analysis (relative risk 3.416; 95% CI, 2.417–4.829; I2 86.6%) (11). Moreover, a recent cross-sectional study including 1,562 diabetes patients found insulin treatment associated with severity of DR (P=0.001) (19) similarly like us. Admittedly, insulin therapy is linked to DR, DME and potentially to DR severity, but the precise mechanistic link is questionable as yet.
Current insights uniquely provided by in vitro studies point out vascular endothelial growth factor (VEGF) signalling in retinal microvascular endothelial cells as the hallmark in the pathophysiology of insulin-associated DR (11,20). Indeed, an in vitro study by Meng et al. (20) demonstrated that insulin interacts with the NADPH oxidase subunit 4 (Nox-4) to induce excess production of reactive oxygen species (ROS) and subsequently oxidative stress in the retinal endothelial cells. ROS produced by insulin are involved in hypoxia-inducible factor-1α (HIF-1α) activation which in turn leads to the expression of VEGF. The latter molecule mediates angiogenesis, neovascularization, and blood-retinal barrier disruption allowing vascular leakage as well (11,20). Taken together, the onset and worsening of DR and DME attributed to insulin use might result from ROS signalling via activation of HIF-1α and consequential VEGF expression.
Data from this study must not be overlooked given its limitations. Owing to the cross-sectional nature of the study, the associations described may not be causal and a bias might have been encountered in selecting patients and ascertaining history-related variables. With respect to the retrospective nature of the study, there were many missing informations in patients’ files, in particular regarding insulin titration and duration of treatment, blood glucose control parameters (glycaemia, glycated haemoglobin), lipid profile, and the types of OHA along the whole study period. Whilst it is long-term insulin therapy which appears harmful, and those missing parameters are acknowledged confounders. On the other hand, this was a preliminary study which stresses the need to reinforce regular diabetic screening programmes in sub-Saharan African countries while awaiting large-scale longitudinal studies for definite conclusions.
Conclusions
The results from this study suggest that insulin treatment may be associated with DR, DR severity and DME in T2DM patients from a sub-Saharan African country such as Cameroon. This calls for in-depth analysis of the observed associations in the context of large-scale longitudinal studies yielding more accurate estimates and providing a better understanding of the mechanistic link between insulin and DR.
Acknowledgements
We are grateful to the patients for their participation in this research.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The ethical committee of the Faculty of Medicine and Biomedical Sciences of the University of Yaoundé I approved the study protocol and an administrative authorization was obtained from the DGH before the recruitment. This study was carried out with the tenets of the Helsinki declaration. All participants provided written informed consent.
References
- Zhao C, Wang W, Xu D, et al. Insulin and risk of diabetic retinopathy in patients with type 2 diabetes mellitus: data from a meta-analysis of seven cohort studies. Diagn Pathol 2014;9:130. [Crossref] [PubMed]
- Hu L, Li DH. Relationship between modified homeostasis model assessment/correlative serum factors and diabetic retinopathy among type 2 diabetics with insulin therapy in Guangzhou, China. Int J Ophthalmol 2014;7:463-8. [PubMed]
- Wu L, Fernandez-Loaiza P, Sauma J, et al. Classification of diabetic retinopathy and diabetic macular edema. World J Diabetes 2013;4:290-4. [Crossref] [PubMed]
- Ciulla TA, Amador AG, Zinman B. Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies. Diabetes Care 2003;26:2653-64. [Crossref] [PubMed]
- International Diabetes Federation. Diabetes atlas. 3rd ed. Brussels: International Diabetes Federation, 2006.
- Klein R, Klein BE, Moss SE. Epidemiology of proliferative diabetic retinopathy. Diabetes Care 1992;15:1875-91. [Crossref] [PubMed]
- Mbanya JC, Sobngwi E. Diabetes in Africa. Diabetes microvascular and macrovascular disease in Africa. J Cardiovasc Risk 2003;10:97-102. [Crossref] [PubMed]
- Jingi AM, Noubiap JJ, Ellong A, et al. Epidemiology and treatment outcomes of diabetic retinopathy in a diabetic population from Cameroon. BMC Ophthalmol 2014;14:19. [Crossref] [PubMed]
- Jingi AM, Nansseu JR, Noubiap JJ, et al. Diabetes and visual impairment in sub-Saharan Africa: evidence from Cameroon. J Diabetes Metab Disord 2015;14:21. [Crossref] [PubMed]
- Kuo JZ, Guo X, Klein R, et al. Association of fasting insulin and C peptide with diabetic retinopathy in Latinos with type 2 diabetes. BMJ Open Diabetes Res Care 2014;2:e000027. [Crossref] [PubMed]
- Zhang J, Ma J, Zhou N, et al. Insulin use and risk of diabetic macular edema in diabetes mellitus: a systemic review and meta-analysis of observational studies. Med Sci Monit 2015;21:929-36. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264-9, W64.
- Wilkinson CP, Ferris FL 3rd, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003;110:1677-82. [Crossref] [PubMed]
- Cepeda-Nieto AC, Esquivel-Contreras MT, Duran-Iñiguez F, et al. High prevalence of diabetic retinopathy and lack of association with integrin α2 gene polymorphisms in patients with type 2 diabetes from Northeastern Mexico. Exp Ther Med 2015;10:435-444. [PubMed]
- Javadi MA, Katibeh M, Rafati N, et al. Prevalence of diabetic retinopathy in Tehran province: a population-based study. BMC Ophthalmol 2009;9:12. [Crossref] [PubMed]
- Raman R, Ganesan S, Pal SS, et al. Prevalence and risk factors for diabetic retinopathy in rural India. Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic Study III (SN-DREAMS III), report no 2. BMJ Open Diabetes Res Care 2014;2:e000005. [Crossref] [PubMed]
- Thomas RL, Dunstan F, Luzio SD, et al. Incidence of diabetic retinopathy in people with type 2 diabetes mellitus attending the Diabetic Retinopathy Screening Service for Wales: retrospective analysis. BMJ 2012;344:e874. [Crossref] [PubMed]
- Aroca PR, Salvat M, Fernández J, et al. Risk factors for diffuse and focal macular edema. J Diabetes Complications 2004;18:211-5. [Crossref] [PubMed]
- Rasoulinejad SA, Hajian-Tilaki K, Mehdipour E. Associated factors of diabetic retinopathy in patients that referred to teaching hospitals in Babol. Caspian J Intern Med 2015;6:224-8. [PubMed]
- Meng D, Mei A, Liu J, et al. NADPH oxidase 4 mediates insulin-stimulated HIF-1α and VEGF expression, and angiogenesis in vitro. PLoS One 2012;7:e48393. [Crossref] [PubMed]


