Effectiveness of enteral feeding protocol on clinical outcomes in critically ill patients: a study protocol for before-and-after design
Introduction
Nutrition therapy is of paramount importance for critically ill patients, because critical illness is usually associated with catabolic state that energy requirements are increased. The term “nutrition support” has been changed to “nutrition therapy”, indicating increased awareness of the importance of nutrition for the critically ill in the medical community. Nutrition can be delivered enterally or intravenously. There is large body of evidence favoring enteral nutrition (EN) to parenteral nutrition (PN) (1). PN is associated with nosocomial infection and prolonged intensive care length of stay, but not mortality (2,3). The most-updated nutrition support guideline recommends that EN should be started within 24 to 48 hours after admission, while PN can be withheld for seven days depending on the risk of malnutrition (4).
Despite the importance of early initiation of EN, it is reported that energy requirements of critically ill patients are far from being reached (5), mainly due to delayed initiation of EN (6). Underfeeding is associated with detrimental clinical outcomes including prolonged length of stay, infection, financial cost, impaired wound healing, and increased morbidity and mortality (5,7). Factors associated inadequate enteral feeding include delayed initiation of EN, slow advancement of infusion rate, gastrointestinal dysfunction, underprescription, incomplete delivery of prescribed nutrition, and frequent interruption of EN (5,8). Some of these factors can be improved with enteral feeding protocols, therefore preventing underfeeding of critically ill patients. There was evidence that implementation of enteral feeding protocol was associated with more EN intake alone, and early initiation of EN (9-11). However, there is no evidence suggesting the reduction of mortality or other patient-important outcomes. The present study aimed to investigate whether enteral feeding protocol was able to improve patient important-outcomes.
Methods
Setting and study population
The study was conducted in intensive care units (ICUs) of ten tertiary care academic centers. Patients admitted to ICU and are expected to stay in ICU for over three days were potentially eligible.
Exclusion criteria include:
- Subjects receiving EN in previous 7 days;
- Contraindications for nasogastric or nasoenteric tube placement;
- Subjects who have already undergone percutaneous endoscopic jejunostomy (PEJ), percutaneous endoscopic gastrostomy (PEG) and surgical jejunostomy;
- Age younger than 18 years old;
- Women who are pregnant or undergo breast feeding;
- Burn patients.
Study design
A before-and-after study design was used, which comprised three phases (12). The first phase is the period without enteral feeding protocol. Participating centers were allowed to deliver EN under the discretion of the treating physician. The second phase was educational phase during which site investigators were gathered and trained for implementation of standardized EN feeding protocol (see below for details). The training program lasts for four weeks and tests will be performed to ensure the trainees can successfully perform the protocol. The last phase is to perform the protocol in participating centers. The site investigators comprised nurses, fellow physicians and dietitians. Compliance to the study protocol is monitored and promoted by a designated investigator. The study was approved by ethics committee of all participating centers (approval No. 2016JS001). The study was registered at International Standard Registered Clinical/soCial sTudy Number (ISRCTN) registry (ISRCTN10583582).
Enteral feeding protocol
Patient evaluation
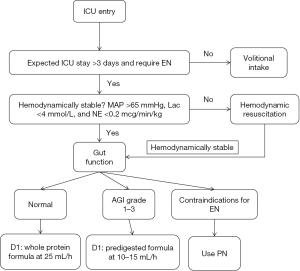
On ICU entry, subjects were evaluated for whether they will stay in ICU for over 3 days and require nasogastric or nasoenteric tube (Figure 1). Subjects are excluded if either of these two conditions is not met. Thereafter, hemodynamic stability is evaluated by three items: (I) mean arterial pressure (MAP) >65 mmHg; (II) serum lactate <4 mmol/L and norepinephrine <12.5 mcg/min. Hemodynamic resuscitation is instituted if the subject is unstable. Hemodynamically stable subjects are then evaluated for the gastrointestinal (GI) function. Normal GI function dictates initiation of whole protein formula at rate of 25 mL/h. For subjects with grade I to III acute gastrointestinal injury (AGI), trophic feeding with predigested formula is delivered at rate of 10–15 mL/h. If there are contraindications for EN including bowel ischemia, bowel perforation/obstruction, proximal fistula and AGI-IV, PN is initiated. Details of AGI grading is shown in Table 1 (13).

Full table
Schematic progressive feeding protocol
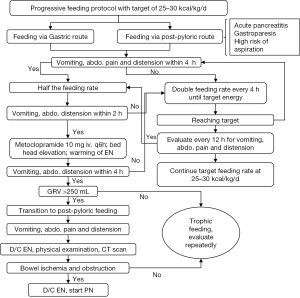
Progressive feeding protocol is performed to target an energy requirement of 25 to 30 kcal/kg/d (Figure 2) (4). Nasogastric tube can be used for those without contraindications. However, for subjects with contraindications (e.g., acute pancreatitis, gastroparesis, high risk of aspiration) to EN administration via nasogastric tube, post-pyloric feeding is considered (14-16). Subjects are then observed for the presence of vomiting, abdominal pain and distension. If present, feeding rate is reduced by 50% and the patient is observed for another 2 hours. If the abdominal signs and symptoms are still present, 10 mg metoclopramide is given intravenously for every 6 hours with bed head elevation and warning of EN. Gastric residual volume (GRV) is measured for every 4 hours. Post-pyloric feeding is initiated if GVR is greater than 250 mL. If abdominal symptoms persist despite these measures, EN is discontinued and comprehensive physical examination and computed tomography is ordered for possible bowel ischemia and obstruction. The presence of these severe conditions will preclude use of EN and PN can be initiated. If GI function improves with intervention, EN can be increased until energy requirement is reached.
Monitoring energy intake
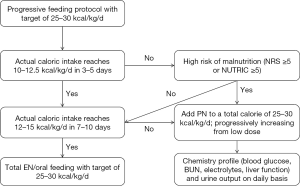
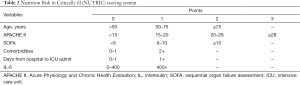
Energy intake should be monitored in the management of critically ill patients. The target is set at 25–30 kcal/kg/day according to the most recent guideline (4). In the first 3–5 days, if the actual calories intake does not reach 10–12.5 kcal/kg/day and the patient is at high risk of malnutrition, PN can be added to a total calorie of 25–30 kcal/kg/day. If calories intake reaches 10–12.5 kcal/kg/day during the first 3–5 days and 12–15 kcal/kg/day during 7–10 days, PN is not required (Figure 3). NRS and NUTRIC scoring system is displayed in Figure 4 and Table 2 (17-19).

Full table
Management of EN tolerance
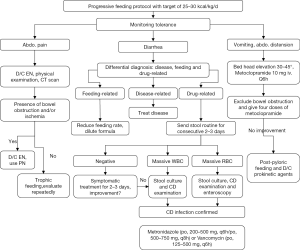
The tolerance to EN is continuously monitored and appropriate interventions are performed (Figure 5) (20). If abdominal pain occurs during EN, physical examination and CT scan will be performed to exclude bowel ischemia and obstruction. The presence of bowel ischemia and obstruction dictates discontinuation of EN. Otherwise, trophic feeding continues and abdominal pain is observed. Differential diagnosis of diarrhea should be done. Common causes of diarrhea in ICU include enteral feeds, diseases and drugs (21). Feeding-related diarrhea can be managed by reducing feeding rate and diluting nutrition formula. Underlying diseases causing diarrhea should be treated. Stool routine should be ordered for drug-related diarrhea. If the test is negative, symptomatic treatment will be enough. If the test shows massive white blood cell (WBC) and/or red blood cell (RBC), further stool culture and examination for Clostridium difficile (CD) will be performed. If CD is confirmed, metronidazole (po, 200–500 mg, q6h/po, 500–750 mg, q8h) or vancomycin (po, 125–500 mg, q6h) can be given (22).
Data collection
A custom-made case report form (CRF) is used for data collection. Data on demographics, comorbidity, organ function, physiological variables, vasopressor use, mechanical ventilation, ventilator setting, AGI grade, chemistry profile, blood routine, blood gas, inflammatory biomarkers (C-reactive protein and procalcitonin), stool routine and culture are recorded. These variables are recorded for the first 7 days after ICU admission, until death or ICU discharge.
Follow up and outcomes
Patients are followed up for 28 days after ICU entry. The primary outcome is 28-day mortality. Secondary outcomes include the proportion of patients with EN, PN and EN + PN, The proportion EN initiation within 24 and 48 hours after ICU entry, proportion of patients who have 60% energy requirement via EN in 3 days, proportion of patients who have 80% energy requirement via EN in 5 days, ICU length of stay, duration of mechanical ventilation, nutritional status and immunity on 7 and 28 days and nosocomial infection.
Sample size calculation
Based on previous observation in participating centers, we assume that the baseline mortality risk without enteral feeding protocol is 50% (23,24). The enteral feeding protocol is able to reduce the mortality by 15%. The study design will provide 80% power and 2.5% (1-sided) type I error. As a result, the fixed sample size is 339. To allow for a small fraction of loss to follow-up, we plan to enroll 350 patients, 175 for each of phases I and III.
Statistical analysis
Continuous data of normal distribution are expressed as mean (± standard deviation) and compared using t test. Skewed data are expressed as median (interquartile range) and compared using Wilcoxon-Mann-Whitney test (25). Categorical variables will be expressed as the number and percentage, and compared using chi-square test.
Because this is a non-randomization design, the confounders cannot be fully controlled. Post hoc analysis with multivariable regression model will be performed by incorporating confounders such as the severity of illness, age, organ dysfunction, and other relevant variables. Initially, all variables will be included to build a full model. Then stepwise backward elimination and forward selection approach will be employed to retain only important variables (26). However, the group variable (with or without enteral feeding protocols) is retained in the model. Subgroup analysis will be performed by restricting to patients with mechanical ventilation. All statistical analysis will be performed using R software (version 3.2.3). A two-sided P<0.05 is considered statistically significant.
Discussion
Although enteral feeding is recommended by international guidelines as the first choice of nutritional therapy for critically ill patients (4,27,28), studies have shown that the energy requirement achieved by EN is far from being reached (29-31). In mechanically ventilated patients, about 66% of patients achieved 80% of caloric requirements within 3 days (32). The figure varies depending on different settings and study populations (33-35). Also, there is large body of evidence showing that underfeeding is associated with significantly increase risk of death (36-38). Although some investigators propose the implementation of permissive underfeeding in critically ill patients, the guideline still recommend early initiation of EN feeding and the goal of 25–30 kcal/kg/day should be achieved (4,39,40). There are reasons that may interfere with the adequate delivery of EN such as delayed initiation of EN, EN cessation for procedures, gastric dysfunction, and diarrhea (6,31,41,42). Therefore, an enteral feeding protocol is mandatory to circumvent risks brought by underfeeding. The study aims to provide a comprehensive enteral feeding protocol that is adapted for ICUs in tertiary care centers in Zhejiang province. The study involves a four-week training program and principal investigators from ten participating centers will be trained. Enteral feeds and clinical outcomes before and after implementation of the protocol will be compared to see its effectiveness.
There are various enteral feeding protocols used in clinical practices and researches. The protocol used in Doig’s study repeatedly assessed whether the energy requirement reached 80% of total goal (10). The goal was not explicitly described and might be set differently by participating centers. In our study we will use the simplistic weight-based equation (25–30 kcal/kg/day) because this is simple that compliance to the protocol can be improved. Although indirect calorimetry (IC) is recommended to determine energy requirement, not all participating centers have equipped with IC (4). Furthermore, Goig’s study did not assess risk of malnutrition, and PN was started if EN is contraindicated. Because the use of PN may have different consequences depending on baseline nutritional status, our study uses NRS and NUTRIC to triage patients who may benefit from PN (Figure 3). Heyland and colleagues conducted an observational study comparing effectiveness of enteral feeding protocol. Participating centers were divided by the presence or absence of feeding protocol. No implementation was performed. Details of feeding protocols were not reported and might vary substantially across participating centers (9). The same study group proposed a feeding protocol called “The Enhanced Protein-Energy Provision via the Enteral Route in Critically Ill Patients” (PEP uP) protocol. The advantage of PEP uP protocol was that it allows nurses to adjust the feeding rate to compensate for procedural interruption. For example, a patient had received 400 mL of 1,500 mL total goal of a day, then feeds were interrupted for several hours while the patient underwent a procedure and there remained nine hours in the day, the new rate would be (1,500−400) mL/9 hr =122 mL/hr for the remaining 9 hr (43). In our protocol, the compensation for procedural interruption is achieved by assessing abdominal tolerance for every 6 hours, and feeding rate can be doubled in the absence of vomiting, abdominal pain and distension.
In conclusion, the article proposes an enteral feeding protocol that is developed according to international practice guidelines and local practices. We plan to conduct a province-wide training program. Participating centers are all the leading medical centers in their cities. The standardized program will bridge the gap between guideline requirement and clinical practice. Furthermore, the study will test the hypothesis that use of enteral feeding protocol is able to improve patient-important outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by ethics committee of all participating centers (approval No. 2016JS001).
References
- Heidegger CP, Darmon P, Pichard C. Enteral vs. parenteral nutrition for the critically ill patient: a combined support should be preferred. Curr Opin Crit Care 2008;14:408-14. [Crossref] [PubMed]
- Elke G, van Zanten AR, Lemieux M, et al. Enteral versus parenteral nutrition in critically ill patients: an updated systematic review and meta-analysis of randomized controlled trials. Crit Care 2016;20:117. [Crossref] [PubMed]
- Netto R, Mondini M, Pezzella C, et al. Parenteral Nutrition Is One of the Most Significant Risk Factors for Nosocomial Infections in a Pediatric Cardiac Intensive Care Unit. JPEN J Parenter Enteral Nutr 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Taylor BE, McClave SA, Martindale RG, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). Crit Care Med 2016;44:390-438. [Crossref] [PubMed]
- Kim H, Stotts NA, Froelicher ES, et al. Why patients in critical care do not receive adequate enteral nutrition? A review of the literature. J Crit Care 2012;27:702-13. [Crossref] [PubMed]
- Turner P. Providing optimal nutritional support on the intensive care unit: key challenges and practical solutions. Proc Nutr Soc 2010;69:574-81. [Crossref] [PubMed]
- Caccialanza R, Klersy C, Cereda E, et al. Nutritional parameters associated with prolonged hospital stay among ambulatory adult patients. CMAJ 2010;182:1843-9. [Crossref] [PubMed]
- Oshima T, Pichard C. Parenteral nutrition: never say never. Crit Care 2015;19 Suppl 3:S5. [PubMed]
- Heyland DK, Cahill NE, Dhaliwal R, et al. Impact of enteral feeding protocols on enteral nutrition delivery: results of a multicenter observational study. JPEN J Parenter Enteral Nutr 2010;34:675-84. [Crossref] [PubMed]
- Doig GS, Simpson F, Finfer S, et al. Effect of evidence-based feeding guidelines on mortality of critically ill adults: a cluster randomized controlled trial. JAMA 2008;300:2731-41. [Crossref] [PubMed]
- Lottes Stewart M. Nutrition support protocols and their influence on the delivery of enteral nutrition: a systematic review. Worldviews Evid Based Nurs 2014;11:194-9. [Crossref] [PubMed]
- Sedgwick P. Before and after study designs. BMJ 2014;349:g5074. [Crossref] [PubMed]
- Reintam Blaser A, Malbrain ML, Starkopf J, et al. Gastrointestinal function in intensive care patients: terminology, definitions and management. Recommendations of the ESICM Working Group on Abdominal Problems. Intensive Care Med 2012;38:384-94. [Crossref] [PubMed]
- Niv E, Fireman Z, Vaisman N. Post-pyloric feeding. World J Gastroenterol 2009;15:1281-8. [Crossref] [PubMed]
- Alkhawaja S, Martin C, Butler RJ, et al. Post-pyloric versus gastric tube feeding for preventing pneumonia and improving nutritional outcomes in critically ill adults. Cochrane Database Syst Rev 2015.CD008875. [PubMed]
- Zhang Z, Xu X, Ding J, et al. Comparison of postpyloric tube feeding and gastric tube feeding in intensive care unit patients: a meta-analysis. Nutr Clin Pract 2013;28:371-80. [Crossref] [PubMed]
- Kondrup J, Rasmussen HH, Hamberg O, et al. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr 2003;22:321-36. [Crossref] [PubMed]
- Heyland DK, Dhaliwal R, Jiang X, et al. Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Crit Care 2011;15:R268. [Crossref] [PubMed]
- Rahman A, Hasan RM, Agarwala R, et al. Identifying critically-ill patients who will benefit most from nutritional therapy: Further validation of the "modified NUTRIC" nutritional risk assessment tool. Clin Nutr 2016;35:158-62. [Crossref] [PubMed]
- Heidegger CP, Graf S, Perneger T, et al. The burden of diarrhea in the intensive care unit (ICU-BD). A survey and observational study of the caregivers' opinions and workload. Int J Nurs Stud 2016;59:163-8. [Crossref] [PubMed]
- Tirlapur N, Puthucheary ZA, Cooper JA, et al. Diarrhoea in the critically ill is common, associated with poor outcome, and rarely due to Clostridium difficile. Sci Rep 2016;6:24691. [Crossref] [PubMed]
- Malamood M, Nellis E, Ehrlich AC, et al. Vancomycin Enemas as Adjunctive Therapy for Clostridium difficile Infection. J Clin Med Res 2015;7:422-7. [Crossref] [PubMed]
- Zhang Z, Ni H, Qian Z., et al. Effectiveness of treatment based on PiCCO parameters in critically ill patients with septic shock and/or acute respiratory distress syndrome: a randomized controlled trial. Intensive Care Med 2015;41:444-51. [Crossref] [PubMed]
- Zhang Z, Zhou J, Shang Y, et al. Effectiveness of anisodamine for the treatment of critically ill patients with septic shock (ACIdoSIS study): study protocol for randomized controlled trial. Ann Transl Med 2015;3:246. [PubMed]
- Zhang Z. Univariate description and bivariate statistical inference: the first step delving into data. Ann Transl Med 2016;4:91. [Crossref] [PubMed]
- Zhang Z. Variable selection with stepwise and best subset approaches. Ann Transl Med 2016;4:136. [Crossref] [PubMed]
- McClave SA, Martindale RG, Vanek VW, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2009;33:277-316. [Crossref] [PubMed]
- Warren M, McCarthy MS, Roberts PR. Practical Application of the Revised Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: A Case Study Approach. Nutr Clin Pract 2016;31:334-41. [Crossref] [PubMed]
- Czapran A, Headdon W, Deane AM, et al. International observational study of nutritional support in mechanically ventilated patients following burn injury. Burns 2015;41:510-8. [Crossref] [PubMed]
- Binnekade JM, Tepaske R, Bruynzeel P, et al. Daily enteral feeding practice on the ICU: attainment of goals and interfering factors. Crit Care 2005;9:R218-25. [Crossref] [PubMed]
- McClave SA, Sexton LK, Spain DA, et al. Enteral tube feeding in the intensive care unit: factors impeding adequate delivery. Crit Care Med 1999;27:1252-6. [Crossref] [PubMed]
- Yip KF, Rai V, Wong KK. Evaluation of delivery of enteral nutrition in mechanically ventilated Malaysian ICU patients. BMC Anesthesiol 2014;14:127. [Crossref] [PubMed]
- Barr J, Hecht M, Flavin KE, et al. Outcomes in critically ill patients before and after the implementation of an evidence-based nutritional management protocol. Chest 2004;125:1446-57. [Crossref] [PubMed]
- De Jonghe B, Appere-De-Vechi C, Fournier M, et al. A prospective survey of nutritional support practices in intensive care unit patients: what is prescribed? What is delivered? Crit Care Med 2001;29:8-12. [Crossref] [PubMed]
- Heyland DK, Dhaliwal R, Wang M, et al. The prevalence of iatrogenic underfeeding in the nutritionally 'at-risk' critically ill patient: Results of an international, multicenter, prospective study. Clin Nutr 2015;34:659-66. [Crossref] [PubMed]
- Villet S, Chiolero RL, Bollmann MD, et al. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr 2005;24:502-9. [Crossref] [PubMed]
- Elke G, Wang M, Weiler N, et al. Close to recommended caloric and protein intake by enteral nutrition is associated with better clinical outcome of critically ill septic patients: secondary analysis of a large international nutrition database. Crit Care 2014;18:R29. [Crossref] [PubMed]
- Mehta NM, Bechard LJ, Cahill N, et al. Nutritional practices and their relationship to clinical outcomes in critically ill children--an international multicenter cohort study*. Crit Care Med 2012;40:2204-11. [Crossref] [PubMed]
- Casaer MP, Van den Berghe G. Editorial on the original article entitled "Permissive underfeeding of standard enteral feeding in critically ill adults" published in the New England Journal of Medicine on June 18, 2015. Ann Transl Med 2015;3:226. [PubMed]
- Weijs PJ. Issues of energy and protein feeding in critically ill: the permissive underfeeding trial. J Thorac Dis 2015;7:E209-11. [PubMed]
- Keehn A, O'Brien C, Mazurak V, et al. Epidemiology of interruptions to nutrition support in critically ill children in the pediatric intensive care unit. JPEN J Parenter Enteral Nutr 2015;39:211-7. [Crossref] [PubMed]
- Passier RH, Davies AR, Ridley E, et al. Periprocedural cessation of nutrition in the intensive care unit: opportunities for improvement. Intensive Care Med 2013;39:1221-6. [Crossref] [PubMed]
- Heyland DK, Cahill NE, Dhaliwal R, et al. Enhanced protein-energy provision via the enteral route in critically ill patients: a single center feasibility trial of the PEP uP protocol. Crit Care 2010;14:R78. [Crossref] [PubMed]


