Immunotherapy in non-small cell lung cancer: the clinical impact of immune response and targeting
Introduction
Lung cancer is one of the most commonly diagnosed malignancies in the world, with 1.8 million new cases in 2012, corresponding to a 12.9% of the global cancer incidence (1). Non-small cell lung cancer (NSCLC) accounts for approximately 85% of all cases (2) and is diagnosed as locally advanced or metastatic at presentation in 70% of patients (3). For patients with advanced-stage NSCLC, chemotherapy with a platinum-doublet offers a median overall survival (OS) of 10 months (4). Recent introduction of molecularly targeted therapies in metastatic disease resulted in clinically meaningful OS improvements, but only in selected patients whose tumors exhibit specific oncogene addiction (5). Therefore, even with the latest advances, lung cancer remains a disease with dismal prognosis, and novel therapies with innovative mechanisms of action are urgently needed.
Immunotherapy refers to a broad class of treatment modalities designed to elicit immune-mediated destruction of tumor cells (TC) (6). Immunotherapy’s encouraging results in other human malignancies hold promise for lung neoplasms, which are considered to be particularly immunogenic and especially those of squamous-cell histology (7). Herein, we aim to provide a comprehensive review about the current understanding of the role of immunotherapy in NSCLC.
General principles
The aim of immunotherapy is to specifically enhance the immune response directed to the tumor. It can be divided into two approaches: active and passive immunotherapy. Passive immunotherapy uses in vitro synthesized immunologic effectors, such as cytokines or immunomodulating monoclonal antibodies, whereas active immunotherapy aims to stimulate immune cells (IC) in vivo, using IC or mediators capable of activating the immune system, such as antitumor vaccines or cellular therapies (8). A list of currently used investigational immunotherapies for lung cancer is provided in the following sections.
Cytokines
The first immunotherapies developed for NSCLC were recombinant cytokines, namely those secreted by Th1 cells, like interleukin (IL)-2 and interferon (IFN). Initial phase II trials were not indicative of clinical benefit for human recombinant IL-2 administration (with or without IFN) (9). In fact, therapy was not well-tolerated, yielding grade 3–4 cardiac and pulmonary toxicity. A subsequent phase II trial by Correale et al. showed that the addition of IL-2 to chemotherapy (gemcitabine plus docetaxel) in patients with advanced NSCLC improved response rates (58.3% vs. 28.6%) with good tolerability (10). However, these findings were not replicated in a phase III randomized trial of IL-2 in combination with chemotherapy with a cisplatin doublet (11). These results were further challenged by a subsequent study reporting a 20% partial response and 50% stable disease among 20 advanced NSCLC patients when IL-2 was administered with the pineal neuro-hormone melatonin (12).
Antitumor vaccines
Antitumor vaccines use the patient’s own immune-surveillance mechanism to induce immune responses against the tumor. This is achieved through the administration of immunogenic tumor-associated antigens or cells in conjunction with an immunoadjuvant that potentiates the immune response.
MAGE-A3 vaccine
MAGE-A3 is a protein almost exclusively expressed by malignant cells (the only normal tissues that express it are the testis and the placenta) and has been documented in 35–50% of NSCLCs (13). In a phase II trial Vansteenkiste et al. randomized 182 completely-resected, MAGE-A3 positive, stage Ib/II NSCLC patients to receive the MAGE-A3 vaccine versus placebo (2:1) (14). There was a 25% relative risk for relapse reduction after a median post-resection period of 44 months, but with non-significant benefits for OS or progression-free survival (PFS) (14). A phase III study is currently underway.
Tecetomide
Tecetomide (L-BLP25) is a liposomal vaccine with the exposed peptide core of mucin 1 (MUC1) (8), which is overexpressed by 86% of lung adenocarcinomas (9). A phase II trial randomized 171 patients with stable/responding stage IIIB/IV NSCLC after any first-line chemotherapy to receive maintenance therapy with tecetomide (enhanced with one single dose of cyclophosphamide) versus best supportive care (BSC) (15). An updated survival analysis showed a significant increase in median 3-year OS (31% vs. 17%, P=0.035), with the greatest difference seen in patients with stage IIIB locoregional disease (16).
In a recently published phase III trial (START), 1,513 patients with unresectable NSCLC, previously responding/stable to chemoradiotherapy, were randomized (2:1) to receive tecetomide versus placebo. Median OS was significantly improved by tecetomide in patients who previously received sequential chemoradiotherapy (30.8 vs. 20.6 months, P=0.012), but not in those who received concurrent chemoradiotherapy (17). However, results are still premature for the clinical practice.
CIMAvax vaccine
EGFR-targeted therapies (e.g., gefitinib and erlotinib) have shown significant results in NSCLC, thus a vaccine therapy targeting EGF has been developed. CIMAvax contains human recombinant EGF conjugated to the P64K Neisseria meningitidis’ carrier protein. It was developed in Cuba and has been tested thoroughly in phase I/II trials (9). Particularly, Neninger et al. (18) (a phase II trial) randomized 80 stage IIIb/IV NSCLC patients to receive either CIMAvax or BSC after first-line chemotherapy completion. A non-significant trend towards better OS with CIMAvax was observed (12.7 vs. 8.5 months). CimAvax is approved in Cuba, Venezuela and Peru for 2nd line treatment of advanced NSCLC (9,13). A phase III study is being carried out outside the United States.
Ganglioside vaccines
One of the earliest attempts for an antitumoral vaccine against NSCLC used the GD3 ganglioside as antigen and the bacille Calmette-Guérin (BCG) as immunoadjuvant. Following a very promising pilot study (19), this BEC2/BCG vaccine didn’t demonstrate a survival or quality of life benefit in a phase III trial with 515 patients conducted by the European Organization for the Research and Treatment of Cancer (EORTC 08971-08971B; Silva Study) (20).
A similar vaccine, named racotumomab, consists of a monoclonal antibody (mAb) that mimics gangliosides with a glycosilation pattern almost exclusive of neoplastic cells. Racotumomab was given to 71 patients with NSCLC in a compassionate use study. The 1-year survival rate was 34% and the OS was 9.9 months (21). Recently, a phase III trial showed a higher median OS of 8.2 months when compared to placebo (6.8 months), P=0.004 (22).
Tumor cell vaccines
Tumor cell vaccines consist of malignant cells harvested from a patient’s tumor, which are subsequently processed and administered to the same patient (autologous vaccines) or another patient (allogeneic vaccines) in order to stimulate cytotoxic immune responses to a similar tumor cell type.
An autologous vaccine, named GVAX, was isolated from 49 NSCLC patients in a phase I/II trial. Seven patients experienced stable disease during 12 weeks or more following first vaccination, but no patients attained a remission (complete or partial) (23).
Belangenpumatucel-L, on the other hand, is an allogeneic vaccine that targets transforming growth factor β2 (TGF-β2). It is produced from four NSCLC cell lines by transfecting them with a plasmid vector with the TGF-β2 gene (8). A phase II trial by Nemunaitis et al. randomized 75 patients with stage II/III/IV NSCLC (1:1:1) to receive Belangenpumatucel-L in different doses. OS was significantly better in the low-dose than in the high-dose cohorts (581 vs. 282 days, respectively). A partial response was reported in 15% of the patients (24).
Dendritic cell (DC) vaccines
DC-based vaccines work by administering activated autologous DCs to the patient, producing a specific immune response against cancer cells. In a phase III trial, a significantly lower recurrence rate was demonstrated in patients treated with surgery and an adjuvant DC vaccine than in patients treated with surgery alone (10% vs. 25%, respectively) (25).
NK cell-related therapies
NSCLC cells evade natural killer cells (NKCs) by expressing certain killer cell immunoglobulin-like receptors (KIR) that inhibit killer cell action. Lirilumab is a fully human mAb specific against certain inhibitory KIRs. It has demonstrated efficacy in preclinical trials, when in combination with nivolumab (26). Phase I trials of lirilumab in combination with immune checkpoint-related mAbs (nivolumab and ipilimumab) are currently underway in solid tumors, including NSCLC patients.
Talactoferrin-a
Talactoferrin-α is a recombinant form of human lactoferrin that binds to the gut epithelium, stimulating the digestive mucosa DCs and thus, the immune system via activation of the immunosurveillance mechanism (27). Digumarti et al. showed that, when given in conjunction with carboplatin/paclitaxel, talactoferrin-α increased response rates in stage IIIb/IV NSCLC patients when compared with placebo chemotherapy (47% vs. 29%, P=0.05) (28). Parikh et al. on the other hand, showed that oral talactoferrin-α in monotherapy significantly increased OS compared to placebo (6.1 vs. 3.7 months, one-tailed log-rank, P=0.040) in a randomized phase II trial (29). However, a recently reported randomized phase III trial (FORTIS-M trial) didn’t show a statistically significant benefit in OS, PFS or response rate for talactoferrin-α compared to placebo (27).
Checkpoint inhibitors in NSCLC
Anti-cytotoxic lymphocyte antigen 4 (CTLA-4) blockade
CTLA-4 blockade has been used either as monotherapy, or in the context of a chemo-immunotherapy approach that combines the cytotoxic effects of chemotherapy with agents modulating the host immune response to the tumor. The hypothesis behind this combination is that tumor cell death triggered by chemotherapy provokes the release of tumor antigens and the subsequent immune response synergizes with and contributes to the success of the cytotoxic treatment (13). As a proof of this concept, in preclinical mouse models, CTLA-4 blockade in combination with various chemotherapeutic agents exhibited a synergistic effect that induced tumor regression and elicited prolonged anti-tumor responses (12).
Ipilimumab (Yervoy®, Bristol-Myer-Squibb) is a fully humanized mAb against the CTLA-4 epitope that neutralizes the receptor, thus enabling cytotoxic T cell activity and perpetuating immune responses (Figure 1). It was first approved for the treatment of metastatic melanoma (13) and is now under clinical development in a number of solid tumors, including NSCLC. A randomized phase II study (30) evaluated the combination of ipilimumab with standard chemotherapy, by randomly assigning 204 patients with stage IIIB/IV NSCLC in a 1:1:1 ratio to receive a “concurrent” ipilimumab regimen (four doses of ipilimumab 3 mg/kg plus carboplatin/paclitaxel followed by two doses of placebo plus carboplatin/paclitaxel), a “phased” ipilimumab regimen (two doses of placebo plus carboplatin/paclitaxel followed by four doses of ipilimumab plus carboplatin/paclitaxel), or a control regimen, comprising up to six cycles of carboplatin/paclitaxel plus placebo. Patients who tolerated the treatment and had no evidence of progression were allowed to receive either ipilimumab or placebo for another 12 weeks (four cycles). The primary end-point of the study, immune-related progression-free survival (irPFS), was met only for the phased ipilimumab versus the control arm [hazard ratio (HR) =0.72; P=0.05] but not for concurrent ipilimumab (HR =0.81; P=0.13). The median irPFS was 5.7, 5.5 and 4.6 months for the phased, concurrent and control arms, respectively. The rates of grade 3 and 4 immune-related AEs were 15%, 20% and 6%, respectively, with two treatment-related deaths reported (one in the concurrent and one in the control arm). A striking element of the study was that immune-related objective response rate (ORR) was nearly doubled in patients treated with phased ipilimumab, as compared to those treated with chemotherapy alone (32% vs. 18%, respectively) (30). Based on these encouraging results, two phase III trials are currently investigating the phased ipilimumab combination against standard chemotherapy (NCT01285609 and NCT02279732).
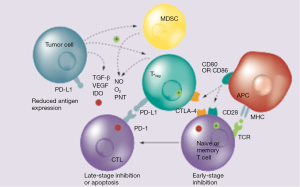
Tremelimumab is another fully human IgG2 mAb against CTLA-4, which is currently in phase III development for malignant melanoma and mesothelioma (31). In a randomized phase II study in 87 patients with pre-treated locally advanced or metastatic NSCLC, PFS at 3 months was not significantly improved by tremelimumab, as compared to BSC (32). Currently, tremelimumab is being evaluated in combination with other immunotherapies, such as MEDI4736 and gefitinib (NCT02040064).
Anti-programmed cell death 1 (PD-1) monoclonal antibodies
Nivolumab
Nivolumab is a fully human IgG4 mAb against the programmed death receptor 1 (PD-1) (Figure 1). In the initial phase I trials with anti-PD-1 antibodies, durable responses and disease stabilization were reported in patients with NSCLC. The pivotal phase I dose-escalation and expansion trial assessed the efficacy and safety of nivolumab in various tumor groups (33). A total of 296 patients were recruited, including patients with NSCLC, melanoma, renal cell cancer, colorectal cancer and prostate cancer. Nivolumab was given intravenously at doses ranging from 0.1 to 10.0 mg/kg every 2 weeks in 8-week cycles. Of note, a maximum tolerated dose (MTD) was not reached due to the very good toxicity profile of this agent. Among the 129 patients with NSCLC (57% with non-squamous histology) who were heavily pre-treated (54% had received ≥3 prior lines), ORR across all doses was 17% and the median OS was 14.9 months for the 3 mg/kg dose. Across all dose levels, 1-, 2- and 3-year survival rates were 42%, 24% and 18%, respectively. Notably, the 1-, 2- and 3-year survival rates for the 3 mg/kg dose were 56%, 42% and 27%, respectively and median duration of response (DOR) was 17 months, which was very promising. The rate of grade 3–4 treatment-related AEs was 14%. Selected treatment-related AEs (any grade) were observed in 41% of the patients with most common being skin (16%), gastrointestinal (12%) and pulmonary events (7%).
Following these results, a single arm phase 2 study was conducted (CheckMate 063); 117 pre-treated patients with advanced squamous NSCLC received nivolumab 3 mg/kg every 2 weeks until PD or intolerable toxicity (34). The primary endpoint of the study was ORR by independent review, which reached 14.5%. Median DOR was not reached [95% confidence interval (CI) 8.31-not applicable] and median PFS and OS were 1.9 and 8.2 months, respectively with a 1-year OS rate of 40.8%. Subsequently, two large phase 3 randomized studies have evaluated nivolumab vs. docetaxel in previously treated advanced squamous (CheckMate 017) (35) and non-squamous (CheckMate 057) (36) patients. Treatment was given until progressive disease, intolerable toxicity or withdrawal of consent. In the CheckMate 017 study, nivolumab at the dose of 3 mg/kg, compared with docetaxel, showed a statistically and clinically significant prolongation of median OS by 3.2 months (9.2 vs. 6.0 months, P<0.001), with a relative 41% reduction in the risk of death. Median PFS (3.5 vs. 2.8 months, P<0.001), ORR (20% vs. 9%, P=0.008) and 1-year OS rate (42% vs. 24%) were also superior in the nivolumab arm. Interestingly, the expression of the PD-1 ligand across all prespecified expression cut-offs (1%, 5%, and 10%), as measured by immunohistochemistry on the tumor-cell membrane, was neither prognostic nor predictive for nivolumab efficacy (35). The positive preliminary results of this trial have already led to the accelerated approval of nivolumab by the U.S. Food and Drug Administration in March 2015, for 2nd line treatment of advanced/metastatic NSCLC of squamous histology that has progressed on or after platinum-based chemotherapy.
The phase III randomized CheckMate 057 trial in non-squamous lung histology, was also stopped after an interim analysis (36). The median DOR was 17.2 and 5.6 months for nivolumab and docetaxel, respectively. At the time of data cut-off, 52% of the patients in the nivolumab arm had ongoing response, while 78% of the patients had quantifiable programmed death ligand 1 (PD-L1) expression. For PD-L1+ tumors, there was an increment in ORR and OS for all three cut-off points, whilst for PD-L1− tumors there was no difference in OS. PD-L1 expression was also predictive for higher responses (range, 31–37%) in the nivolumab arm. Treatment related AEs were more frequent in the docetaxel arm (any grade: 88% vs. 69%; grade 3–4: 54% vs. 10%). The rate of pneumonitis in the nivolumab arm was 3% (grade 3–4: 1%). Further trials are ongoing to evaluate nivolumab versus standard chemotherapy (NCT01642004 and NCT02041533), in combination with chemotherapy (NCT01454102), or as a single agent in a pre-treated population (NCT02066636 and NCT02409368).
Pembrolizumab (MK-3475)
Pembrolizumab, a humanized IgG4 mAb against PD-1, was investigated in a large phase I dose-finding study at doses of 2 mg/kg, 10 mg/kg 3-weekly or 10 mg/kg bi-weekly, across several cohorts of pre-treated and treatment-naïve patients (37). PD-L1 expression was evaluated by IHC on a fresh biopsy sample and only patients with membranous staining ≥1% were eligible. Four hundred and ninety-five patients with NSCLC were allocated to a training group (n=182) or a validation group (n=313). Patients were assessed every 9 weeks until there was confirmed disease progression as per immune-related response criteria (irRC), unacceptable toxicity, or withdrawal of consent. In the overall population, the ORR, PFS and OS were 19.4%, 3.7 and 12 months, respectively. From the training set, a cut-off point of PD-L1 expression of ≥50% of cells (proportion score) was identified and was subsequently assessed in the validation set. Patients with a proportion score of at least 50% (approximately 23% of the evaluable patients) had a higher ORR and longer PFS, OS and DOR compared to the proportion score groups of <1% or 1–49%.
Seventy percent of the patients experienced drug-related AEs (9.5% grade ≥3). The most common side effects were fatigue (19.4%), pruritus (10.7%) and appetite loss (10.5%). Any grade and grade ≥3 pneumonitis were observed in 3.6% and 1.8% of the patients, respectively (37). Randomized phase III studies of pembrolizumab monotherapy versus standard chemotherapy are planned or ongoing in the first- and second-line settings for patients with PD-L1+ NSCLC (NCT02220894 and NCT01905657).
Anti-PD-L1 monoclonal antibodies
Atezolizumab (MPDL3280A)
Atezolizumab (formerly known as MPDL3280A) is a mAb that targets PD-L1 and B7.1, expressed on TC. It has shown promising activity in several neoplasms related to tobacco smoking, such as urothelial bladder cancer. By preventing the blockade of the PD-1/PD-L2 interaction, such antibodies may have a favorable toxicity profile compared with anti-CTLA-4 or anti-PD-1 antibodies. Initial reports from an interim analysis of a phase I expansion study (POPLAR) (38) in pre-treated patients with SqCC and non-SqCC receiving atezolizumab, showed an ORR of 24% and 24-week PFS reaching 48%. The incidence of all grade 3/4 adverse events was 34%. There was a clear difference in ORR according to the PD-L1 status expression, with 100% ORR observed in tumors being PD-L1 positive and 15% ORR in patients with PD-L1 negative tumors. These results led to a breakthrough therapy designation granted to atezolizumab for the treatment of PD-L1 positive NSCLC patients who progressed during or after standard treatments. Of note, PD-L1 expression was evaluated on both tumor and infiltrating IC.
Results of the interim analysis from a phase II trial evaluating atezolizumab vs. docetaxel as second- or third-line treatment have recently been presented (38). This study assessed PD-L1 expression on both TC and infiltrating IC on archival or fresh tissue. In the ITT population, OS was numerically longer for the atezolizumab arm (11.5 vs. 9.5 months, HR =0.77, P=0.11). Improved efficacy was observed with increasing PD-L1 expression (Table 1). Median DOR was not reached and was 7.8 months for the atezolizumab and docetaxel arms, respectively. Toxicity was higher in the docetaxel arm: treatment related AEs (88% vs. 67%) and grade 3–4 treatment related AEs (39% vs. 12%) but there was no difference in toxic death rates (4% in both arms). Several trials are currently ongoing to evaluate atezolizumab in pre-treated advanced NSCLC (NCT01846416) or in the first-line setting (NCT02409342, NCT02367781 and NCT02367794)
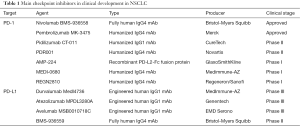
Full table
Durvalumab (MEDI4736)
Durvalumab (previously known as MEDI4736) is an engineered human IgG1 mAb against PD-L1, which has been tested in a phase I/II study in several tumor types (38). Durvalumab was administered at a dose of 10 mg/kg iv every 2 weeks until PD, unacceptable toxicity or up to 12 months. Two hundred and twenty-eight advanced NSCLC patients (126 non-squamous and 102 squamous) have been included in this study (39). Drug-related AEs were reported in 50% (grade 3–4: 8%) of the patients; most commonly fatigue, decreased appetite and nausea. Grade 3–4 drug-related AEs were reported in 8% of the patients and there were no toxic deaths. Pneumonitis (grade 1–2) occurred in 1% of the patients. ORR was 16% (27% in PD-L1+), and disease control rate (DCR) at 12 weeks was 42%. ORR was 21% and 13% for squamous and non-squamous histology, respectively. Sixty-six percent of the patients had ongoing responses (DoR range, 0.1+ to 54.4+ weeks). According to preliminary OS data, patients with PD-L1+ tumors have longer OS than patients with PD-L1− tumors. Further trials are currently ongoing (NCT02087423, NCT02273375, NCT02125461 and NCT02352948).
Immunotherapy combinations and future challenges
The combination of drugs acting through different mechanisms to inhibit/enhance the same process is the basis for immunotherapeutic combinations with synergistic effect. A study in mice showed that, when compared to single checkpoint inhibition, double blockade increased the proliferation of cluster of differentiation (CD)8+ and CD4+ T cells, cytokine release, signalling molecules critical for T cell function and inhibition of regulatory T lymphocyte (Treg) cell suppressive functions (40).
The CA209-012 study was a phase I study comparing nivolumab as monotherapy or combined with chemotherapy, targeted therapy, or ipilimumab in patients with NSCLC (NCT01454102). Interim results of the first-line combination of nivolumab and ipilimumab were presented at the ASCO annual meeting in 2014 (41). The first arm received nivolumab 1 mg/kg and ipilimumab 3 mg/kg and the second arm was given nivolumab 3 mg/kg and ipilimumab 1 mg/kg, for four cycles. Both arms then received nivolumab 3 mg/kg until disease progression or unacceptable toxicity. ORR was 11% and 13% (for patients in the first arm with squamous and non-squamous histology, respectively) and 33% and 13% for the corresponding groups of patients in the second arm. Grade 3–4 AEs were reported in 49% of the patients across both arms. The most common serious AEs were pneumonitis, diarrhea, colitis and elevated AST and ALT enzymes. Three of the 49 patients enrolled died of drug-related toxicities (41).
Ipilimumab is also being studied in combination with anti-PD-1 and anti-PD-L1 antibodies: with pembrolizumab as one of the experimental cohorts in the KEYNOTE-021 study (NCT02039674) and with atezolizumab (NCT02174172). Tremelimumab is currently being evaluated in combination with durvalumab for advanced NSCLC (NCT02000947, NCT02453282 and NCT02352948)
Immunotherapy for lung cancer in the adjuvant setting
Adjuvant therapy in early lung cancer stages is now the standard of care; however, still more than 40% of the patients will relapse and die from metastatic disease. Therefore, novel strategies, such as immunotherapy, are being investigated in the adjuvant setting, in an attempt to eliminate micro metastatic disease post-resection. Among several vaccination strategies that have been studied in the past, MAGE-A3 is an antigen-specific cancer immunotherapy that has been investigated in a large adjuvant program for lung cancer. An initial randomized placebo-controlled phase II study (14) in 182 patients with completely resected NSCLC expressing the MAGE-A3 gene investigated the MAGE-A3 vaccine and resulted in a favorable profile for the vaccine over placebo. This led to a phase III trial, the MAGE-A3 as Adjuvant Non-Small Cell LunG CanceR ImmunoTherapy (MAGRIT) trial; the largest ever phase III lung cancer adjuvant trial with a vaccine for MAGE-A3 expressing, stage IB, II and IIIA NSCLC. The study was initiated in 2007 and enrolled 2,270 patients from 400 centers in 33 countries. In April 2014 the study was prematurely discontinued because it failed to meet its primary endpoint, as it did not show any significant differences in DFS between the MAGE-A3 vaccinated patients versus those on placebo (14). Several other smaller immunotherapy attempts have also failed in the adjuvant setting and more recently a small randomized, controlled phase III study from Japan was presented at the 2015 World Conference on Lung Cancer in Denver, (abstract 04.01), with adjuvant chemo-immunotherapy showing improved survival rates for patients with NSCLC, compared to adjuvant chemotherapy alone. Immunotherapy, in this small but innovative study, comprised adoptive transfer of autologous activated killer T cells and DCs from the patients’ regional lymph nodes (42).
Current challenges in lung cancer immunotherapy
One of the “hottest” topics in lung cancer immunotherapy concerns biomarkers for these newly developed drugs (24,43). Biomarker research has increasingly been identified as one of the main challenges in cancer immunotherapy (44). PD-1 and immunohistochemical PD-L1 expression have been proposed as potential biomarkers for anti-PD-1/PD-L1 activity although they are far from being optimal, since a substantial number of patients with “negative” immunohistochemistry still derive clinical benefit from these agents.
Another concern relates to the definition of response to therapy. Ipilimumab investigation in melanoma showed that in certain cases, immunotherapy response patterns were dissimilar from those of standard therapies, even though there was indeed a response to treatment (12,13). By promoting lymphocyte infiltration and inflammatory edema in the tumor, ipilimumab may transiently increase the lesion size, while maintaining anti-tumoral efficacy. Also, tumor growth continues as the immune response takes time to develop. Response Evaluation Criteria in Solid Tumors (RECIST) are, therefore, not fully adequate to measure responses to ipilimumab. The irRC have been developed to fill this gap. In irRC, the patient’s total tumor burden is calculated and used as baseline for future comparative imaging (12).
Furthermore, we need to define the optimal setting for use of lung cancer immunotherapy: in the adjuvant setting, in first-line, at relapse, or as consolidation or maintenance. The optimal duration of immunotherapy is also another unanswered question.
The introduction of a new therapeutic modality for lung cancer requires the identification and understanding of the unique side effects that the new immunotherapy agents have. Immune-related toxicities are well recognized, with both PD-1/PD-L1 inhibitors and CTLA-4 antibodies, with different rates and severities observed between the two classes of drugs. Vigilance is required for the early assessment and management of specific toxicities in lung cancer patients.
Finally, immunotherapies that exert effects through distinct pathways may act synergistically and a trial with concomitant ipilimumab and nivolumab is currently underway for NSCLC. The proper way to combine these novel immunotherapies with the standard available therapeutic modalities for cancer, such as chemotherapy, targeted therapy, radiotherapy and surgery, is a matter of ongoing research.
Conclusions
Immunotherapy for lung cancer treatment is now a reality (10,13). Monoclonal antibodies targeting immune checkpoints and anti-tumor vaccines are, at present, the most promising components of this therapeutic strategy. Clinical investigation in the field is intense and novel drugs are being rapidly developed and tested. Important clinical trials are currently ongoing in the second and first-line settings. Their results are eagerly awaited, in order to appropriately position immunotherapy in the lung cancer therapeutic algorithm: alone or in combination with other existing treatment modalities. A deeper understanding of the underlying mechanisms of lung cancer immunology, a better definition of the clinical response criteria and the identification of robust biomarkers would certainly be the hallmarks of this exciting field.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- GLOBOCAN 2012. All Cancers (excluding non-melanoma skin cancer) Estimated Incidence, Mortality and Prevalence Worldwide in 2012. Accessed January 4, 2013. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
- Sher T, Dy GK, Adjei AA. Small cell lung cancer. Mayo Clin Proc 2008;83:355-67. [Crossref] [PubMed]
- Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. [Crossref] [PubMed]
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51. [Crossref] [PubMed]
- Jackman DM, Miller VA, Cioffredi LA, et al. Impact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated non-small cell lung cancer patients: results of an online tumor registry of clinical trials. Clin Cancer Res 2009;15:5267-73. [Crossref] [PubMed]
- Hall RD, Gray JE, Chiappori AA. Beyond the standard of care: a review of novel immunotherapy trials for the treatment of lung cancer. Cancer Control 2013;20:22-31. [PubMed]
- McCarthy F, Roshani R, Steele J, et al. Current clinical immunotherapy targets in advanced nonsmall cell lung cancer (NSCLC). J Leukoc Biol 2013;94:1201-6. [Crossref] [PubMed]
- Aerts JG, Hegmans JP. Tumor-specific cytotoxic T cells are crucial for efficacy of immunomodulatory antibodies in patients with lung cancer. Cancer Res 2013;73:2381-8. [Crossref] [PubMed]
- Rijavec E, Genova C, Alama A, et al. Role of immunotherapy in the treatment of advanced non-small-cell lung cancer. Future Oncol 2014;10:79-90. [Crossref] [PubMed]
- Correale P, Tindara Miano S, Remondo C, et al. Second-line treatment of non small cell lung cancer by biweekly gemcitabine and docetaxel +/- granulocyte-macrophage colony stimulating factor and low dose aldesleukine. Cancer Biol Ther 2009;8:497-502. [Crossref] [PubMed]
- Ridolfi L, Bertetto O, Santo A, et al. Chemotherapy with or without low-dose interleukin-2 in advanced non-small cell lung cancer: results from a phase III randomized multicentric trial. Int J Oncol 2011;39:1011-7. [PubMed]
- Lissoni P, Brivio F, Fumagalli L, et al. Neuroimmunomodulation in medical oncology: application of psychoneuroimmunology with subcutaneous low-dose IL-2 and the pineal hormone melatonin in patients with untreatable metastatic solid tumors. Anticancer Res 2008;28:1377-81. [PubMed]
- Monteiro ID, Califano R, Mountzios G, et al. Immunotherapy with checkpoint inhibitors for lung cancer: novel agents, biomarkers and paradigms. Future Oncol 2016;12:551-64. [Crossref] [PubMed]
- Vansteenkiste J, Zielinski M, Linder A, et al. Adjuvant MAGE-A3 immunotherapy in resected non-small-cell lung cancer: phase II randomized study results. J Clin Oncol 2013;31:2396-403. [Crossref] [PubMed]
- Butts C, Murray N, Maksymiuk A, et al. Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J Clin Oncol 2005;23:6674-81. [Crossref] [PubMed]
- Butts C, Maksymiuk A, Goss G, et al. Updated survival analysis in patients with stage IIIB or IV non-small-cell lung cancer receiving BLP25 liposome vaccine (L-BLP25): phase IIB randomized, multicenter, open-label trial. J Cancer Res Clin Oncol 2011;137:1337-42. [Crossref] [PubMed]
- Butts C, Socinski MA, Mitchell PL, et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol 2014;15:59-68. [Crossref] [PubMed]
- Neninger E, Verdecia BG, Crombet T, et al. Combining an EGF-based cancer vaccine with chemotherapy in advanced nonsmall cell lung cancer. J Immunother 2009;32:92-9. [Crossref] [PubMed]
- Grant SC, Kris MG, Houghton AN, et al. Long survival of patients with small cell lung cancer after adjuvant treatment with the anti-idiotypic antibody BEC2 plus Bacillus Calmette-Guérin. Clin Cancer Res 1999;5:1319-23. [PubMed]
- Giaccone G, Debruyne C, Felip E, et al. Phase III study of adjuvant vaccination with Bec2/bacille Calmette-Guerin in responding patients with limited-disease small-cell lung cancer (European Organisation for Research and Treatment of Cancer 08971-08971B; Silva Study). J Clin Oncol 2005;23:6854-64. [Crossref] [PubMed]
- Alfonso S, Diaz RM, de la Torre A, et al. 1E10 anti-idiotype vaccine in non-small cell lung cancer: experience in stage IIIb/IV patients. Cancer Biol Ther 2007;6:1847-52. [Crossref] [PubMed]
- Alfonso S, Valdés-Zayas A, Santiesteban ER, et al. A randomized, multicenter, placebo-controlled clinical trial of racotumomab-alum vaccine as switch maintenance therapy in advanced non-small cell lung cancer patients. Clin Cancer Res 2014;20:3660-71. [Crossref] [PubMed]
- Nemunaitis J, Jahan T, Ross H, et al. Phase 1/2 trial of autologous tumor mixed with an allogeneic GVAX vaccine in advanced-stage non-small-cell lung cancer. Cancer Gene Ther 2006;13:555-62. [Crossref] [PubMed]
- Nemunaitis J, Dillman RO, Schwarzenberger PO, et al. Phase II study of belagenpumatucel-L, a transforming growth factor beta-2 antisense gene-modified allogeneic tumor cell vaccine in non-small-cell lung cancer. J Clin Oncol 2006;24:4721-30. [Crossref] [PubMed]
- Skachkova OV, Khranovska NM, Gorbach OI, et al. Immunological markers of anti-tumor dendritic cells vaccine efficiency in patients with non-small cell lung cancer. Exp Oncol 2013;35:109-13. [PubMed]
- Creelan BC, Antonia SJ. Immunotherapy in lung cancer: "b7-bombers" and other new developments. Semin Respir Crit Care Med 2013;34:810-21. [Crossref] [PubMed]
- Ramalingam S, Crawford J, Chang A, et al. Talactoferrin alfa versus placebo in patients with refractory advanced non-small-cell lung cancer (FORTIS-M trial). Ann Oncol 2013;24:2875-80. [Crossref] [PubMed]
- Digumarti R, Wang Y, Raman G, et al. A randomized, double-blind, placebo-controlled, phase II study of oral talactoferrin in combination with carboplatin and paclitaxel in previously untreated locally advanced or metastatic non-small cell lung cancer. J Thorac Oncol 2011;6:1098-103. [Crossref] [PubMed]
- Parikh PM, Vaid A, Advani SH, et al. Randomized, double-blind, placebo-controlled phase II study of single-agent oral talactoferrin in patients with locally advanced or metastatic non-small-cell lung cancer that progressed after chemotherapy. J Clin Oncol 2011;29:4129-36. [Crossref] [PubMed]
- Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol 2013;24:75-83. [Crossref] [PubMed]
- Chang S, Lin X, Higashikubo R, et al. Unique pulmonary antigen presentation may call for an alternative approach toward lung cancer immunotherapy. Oncoimmunology 2013;2:e23563. [Crossref] [PubMed]
- Winter H, van den Engel NK, Rusan M, et al. Active-specific immunotherapy for non-small cell lung cancer. J Thorac Dis 2011;3:105-14. [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015;16:257-65. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Segal NH, Antonia SJ, Brahmer JR, et al. Preliminary data from a multi-arm expansion study of MEDI4736, an anti-PD-L1 antibody. J Clin Oncol 2014;32:abstr 3002^.
- Rizvi NA, Brahmer JR, Ou SH, et al. Safety and clinical activity of MEDI4736, an anti-programmed cell death-ligand 1 (PD-L1) antibody, in patients with non-small cell lung cancer (NSCLC). J Clin Oncol 2015;33:abstr 8032.
- Stämpfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol 2009;9:377-84. [Crossref] [PubMed]
- NCT01454102. Study of Nivolumab (BMS-936558) in Combination With Gemcitabine/Cisplatin, Pemetrexed/Cisplatin, Carboplatin/Paclitaxel, Bevacizumab Maintenance, Erlotinib, Ipilimumab or as Monotherapy in Subjects With Stage IIIB/IV Non-small Cell Lung Cancer (NSCLC) (CheckMate 012). Available online: https://clinicaltrials.gov/ct2/show/NCT01454102
- Kimura H, Matsui Y, Ishikawa A, et al. Randomized controlled phase III trial of adjuvant chemo-immunotherapy with activated killer T cells and dendritic cells in patients with resected primary lung cancer. Cancer Immunol Immunother 2015;64:51-9. [Crossref] [PubMed]
- Morse MA, Osada T, Hobeika A, et al. Biomarkers and correlative endpoints for immunotherapy trials. Am Soc Clin Oncol Educ Book 2013. [Crossref] [PubMed]
- Fox BA, Schendel DJ, Butterfield LH, et al. Defining the critical hurdles in cancer immunotherapy. J Transl Med 2011;9:214. [Crossref] [PubMed]


