High-sensitivity cardiac troponin testing in routine practice: economic and organizational advantages
Cardiac troponins (cTn) and myocardial infarction (MI)
The troponin C (TnC), I (TnI) and T (TnT) are structural components of the myofilaments that regulate muscle contraction by interacting with calcium and tropomyosin, and inhibiting the ATPase activity of actomyosin. In the late 1980’s a first assay (radioimmunoassay, RIA) for specific detection of cTn was made available, and shortly afterwards several more practical assays based on immunoenzymatic techniques were developed (1,2). The clinical adoption of cTn immunoassays was based on the assumption that the specific antibodies used in the assays were able to distinguish cTnI or cTnT from their skeletal muscle isoforms (2), thus enabling to reliably identify a cardiac myocyte injury. The nearly absolute cardiac specificity of both cTnI and cTnT immunoassays has then allowed to widespread their use as a valuable support for diagnosing MI. Despite their specificity, the genuine qualitative approach to cTn testing based on the rough equivalency “positive cTn equals MI” is erroneous, though still difficult to be erased from many physicians’ attitude. Increased values of cTn are often consequence of myocardiocytes damage, but not necessarily secondary to an ischemic event nor to a specific cardiac disease (3). The obvious consequence of the purely qualitative approach would be a dramatic increase in the number of tentative diagnosis of MI. Indeed, a dynamic view of cTn testing has been suggested before the turn of the century. In 1997 Hamm et al. (4) described the results of an interventional study on 773 consecutive patients with onset of chest pain for less than 12 h and without ST segment elevation. The cTn concentration (both cTnI and cTnT) was assayed by repeated testing, at patient arrival and after 4 h or more. By this approach, cTn values were found to be positive in 16% (cTnT) to 22% (cTnI) of all patients. During a follow-up of 30 days, 20 deaths and 14 nonfatal MIs were recorded, and both cTnI and cTnT were proven to be strong and independent predictors of cardiac outcome, wherein the event rates in patients with negative tests were only 1.1% for cTnT and 0.3% for cTnI.
The greater diagnostic value of cTn compared to other biomarkers (e.g., myoglobin and creatine kinase MB; CK-MB), along with the need of evaluating the biomarker kinetics, were reaffirmed by both the European and American cardiology societies in the 2000 (5,6), concluding that testing on admission and 6–12 h thereafter would provide a better risk stratification than using previous algorithms based only on single testing. Another cornerstone, besides repeat testing, is that test results should be made available within 30–60 minutes (7), because increased cTn values would be helpful for identifying those patients who benefit most from early invasive strategies (5,6).
A further refinement of cTn testing, impacting both the interpretation of results and testing algorithms, was the establishment of reference values to be used as thresholds. Historically, the “cutoff” for cTn assays had been established by receiver-operating characteristics (ROC) curves, as usual in laboratory medicine when evaluating “quantitative” assays. At the eve of the new century, the joint guidelines of the National Academy of Clinical Biochemistry (NACB) and the International Federation of Clinical Chemistry (IFCC) (7) recommend the use of the 99th percentile derived from a healthy population as the decision limit. Notably, it was also affirmed that such value would require a total imprecision (coefficient of variation; CV) ≤10% to make cTn testing suitable for clinical use. The guidelines also enforced the need of collecting additional blood specimens after those drawn on admission, and to guide the diagnosis on the basis of results obtained by sequential sampling. This aspect introduced a paradigm shift in laboratory organization, since most laboratories did not have a mechanism for automatic “reflex testing” i.e., testing entailing the ordering or cancellation of follow-up tests on a given sample based on results of preliminary tests (7).
Unfortunately, none of the cTn commercial assays available at that time complied with the “10% at 99th” requirement, so that all manufacturers were forced to retool their products and market them as “high” or even “ultra” sensitivity (8). Indeed, that was an odd and quite arbitrary definition, wherein a universally agreed criterion for classifying the methods according to their analytical sensitivity was lacking at that time (8). Moreover, the use of new thresholds without a clear understanding and agreement about the clinical interpretation of cTn data led to a substantial increase in the rate of MI diagnosis in the emergency department (ED), especially attributable to the presence of increased cTn values in elderly patients as well as in those with extra-cardiac diseases (e.g., impaired renal function) (8,9). As a consequence, the number of patients referred to cardiac intensive care units or directly to the catheterization lab skyrocketed, thus placing additional workload on those units and generating the uneasy feeling that the so-called “troponinoses” were causing more harm than good in clinical practice. The feelings from many clinicians are well reflected by the celebrated sentence “when troponin was a lousy assay it was a great test, but now that it’s becoming a great assay, it’s getting to be a lousy test” (10). As most of us would agree, the sensitivity of cTn immunoassays should not be considered as important as its global diagnostic performance, which shall ultimately respond to the need of the emergency physicians.
Additional refinements for the appropriate use of cTn in diagnosing ACS have been warranted. In the 2012 two papers of utmost relevance were almost simultaneously published. A joint committee of the major international scientific societies for cardiology released the 3rd universal definition of MI (11), which definitely affirmed the central role of the increase/decrease of cTn values over time for establishing a definitive diagnosis of myocardial ischemic injury. Shortly afterward, a “ad hoc” committee of the IFCC eventually established the criteria to define a “high sensitivity (HS)” assay for cTn (12), as follows:
- Total imprecision (i.e., CV) not exceeding 10% at the decisional value represented by the 99th percentile of a reference healthy population;
- Capability of detecting cTn values in at least 50% of the above mentioned population.
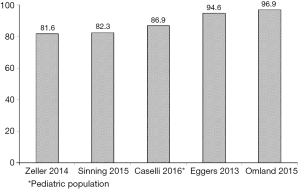
In the same year Apple et al. (13) emphasized that virtually none of the on-market immunoassays was able to fulfill both criteria. The rate of detection of cTn in a “normal” population of 524 presumably healthy North American individuals ranged from 0–35% with the available assays for both cTnI and cTnT, and only one of those was able to detect cTnI in as many as 96% individuals in that cohort. Despite the frequency of detectable cTn in the general population largely depends on selection criteria (14-16), a very high rate of positivity has been observed in ensuing studies carried out in representative samples of the general population (17-20), as well as in the pediatric age (21,22) (Figure 1).
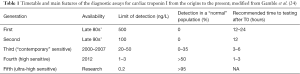
A comprehensive review of the history of cTn has been recently published by Conrad and Jarolim (23), and the characteristics of the different generations of immunoassays are summarized in Table 1. In summary, current evidence suggests considering cTn as a truly quantitative parameter, thus overcoming the former concept of “negative or positive” (i.e., the “black or white” paradigm) results (24). Therefore, the current section can be summarized and concluded with the statement contained in the most recent guidelines of the European Society of Cardiology (ESC) (25): “Cardiac troponin should be interpreted as a quantitative marker: the higher the level, the higher the likelihood for the presence of myocardial infarction”.
Full table
MI in the ED
Now that most of the semantic controversies that plagued the field of cTn testing in ACS should have come to an end, it is time to focus on the real-life impact of HS-cTn immunoassays as a first-line test in one of the most crucial health care settings that is represented by short stay units, especially the ED.
The current workload of EDs has remarkably increased in many countries over the last decade, due to several factors such as lower resources for extra-hospital treatment of acute patients, increase of resident population and enhancement of life expectancy, which then leads to a higher number of elderly people presenting to the ED with acute exacerbations of chronic diseases. It has been estimated that 10–15% of ED patients present with chest pain or other signs suggestive of myocardial ischemia, but a final diagnosis of ACS can only be made in 15–25% of them, which overall represents the 2–5% of all incomers (26-31). The overcrowding of the ED is directly associated with clinical endpoints (e.g., mortality), and also with care processes that bear a high clinical relevance, such as time to initiate treatment for patients in critical conditions or diseases with a potentially severe outcome (32). Specifically, the negative effect of overcrowding has been demonstrated, and was found to be especially relevant for the higher risk of adverse cardiovascular outcomes in patients with both ACS-related and non-ACS-related chest pain syndrome (26). Despite remarkable advances in this field, the misdiagnosis of MI remains a tangible risk. The relevance of using appropriate decision values has been pinpointed by Wildi et al. (33), who demonstrated that a significantly harm, in terms of morbidity and mortality, may occur in patients that are withheld from evidence-based therapies (e.g., rhythm monitoring for 24–48 h, antiplatelet therapy, high-dose statins and early revascularization).
Another peculiar setting is represented by the (mis)diagnosis of MI and the higher mortality for cardiovascular diseases in the female gender. Major inequalities still exist between men and women in treatment and outcome of ACS, since both early and late deaths are considerably higher in women (34,35). Accordingly, cardiovascular disease in the female gender is now regarded as one of the primary targets for activity of the EU-funded project EUGenMed (36). Very recently, the American Heart Association (AHA) has issued a document (37) reaffirming the existence of sex-specific differences in presentation, pathophysiological mechanisms, and outcomes in patients with MI. This evidence was been then confirmed in subsequent analyses, reviewed elsewhere (38,39). Traditionally, the notion that a diagnosis of MI is less likely in women with suspected ACS has been attributed to the less frequency of typical symptoms and the lower frequency of suggestive electrocardiography findings. Women are also less likely to be referred to a cardiologist or undergo coronary revascularization (40). It has been recently demonstrated that cTn may play a substantial role in the female gender. Women have a lower concentration of circulating cTn, and this strongly impacts on the expected “normal” values and the calculation of the 99th percentile. Although this aspect has been known for a long time, even before the development of HS assays, the difference of normal values between genders does not translate - in clinical practice-into different diagnostic criteria because the former, along with the “contemporary” assays (Table 1), were not accurate enough for measuring the low values of cTn commonly found in women. Conversely, the use of a recently developed HS-cTnI test, which displays a functional sensitivity (10% total CV at 5 ng/L) at a much lower level than the 99th percentile in women (i.e., 16 ng/L) (13), would allow a more accurate diagnosis in both genders. The clinical relevance of this approach has been brilliantly demonstrated by a recent study published by Shah et al. (40), including 1,126 consecutive patients with suspected ACS. The novel HS-cTnI immunoassay was employed with the adoption of gender specific thresholds of 34 ng/L for men and 16 ng/L for women and compared with the previous, “contemporary” assay on the same platform and using a common diagnostic threshold of 50 ng/L in both genders. The adoption of gender-specific cutoffs using the HS-cTnI technique was hence effective to generate a considerable improvement in the diagnostic rate of MI in women (i.e., from 11% to 22%), though it produces a more marginal improvement in men (from 19% to 21%).
Besides the diagnosis of an actual ACS, prognostication is also a crucial issue in patients with heart disease. Short-term clinical outcomes, usually at 30-day after admission, are of major relevance in the ED, in order to exploit a safe rule out. A survey carried out in the US indicated that 0.4% of patients discharged directly by the ED died or had a documented MI within 30 days (41). Some recent papers have tried to address this issue in direct relationship to the use of HS-cTn, by evaluating the association between biomarker values at admission and occurrence of major cardiovascular adverse events (MACE) at 30 days. Bohula May et al. (42) investigated the prognostic performance of a HS-cTnI immunoassay for predicting cardiovascular death or new MI at 30 days in 4,695 patients with non-ST elevation MI (NSTEMI) enrolled in two prospective clinical trials (EARLY-ACS and SEPIA-ACS1-TIMI (43). Values of cTnI were detectable at baseline in all patients and, after adjusting for the TIMI (Thrombolysis In Myocardial Infarction) risk score, patients with cTnI values above the non-gender specific 99th percentile (i.e., 26 ng/L) had a 3.7-fold higher adjusted risk of cardiovascular death or MI at 30 days compared to patients with cTnI values lower than the 99th percentile. Notably, a significant difference was found between cTnI and cTnT in this cohort, inasmuch as patients with a negative cTnT value (i.e., <10 ng/L) but with cTnI >26 ng/L were at increased risk of death or MI compared to patients with cTnI values <26 ng/L. Therefore, a very low cTnI level at presentation, that is reliably measurable with HS immunoassays, can identify patients with NSTEMI who have a higher risk of recurrent events of clinical relevance at 30 days.
HS-cTn in the ED: organizational and economic aspects
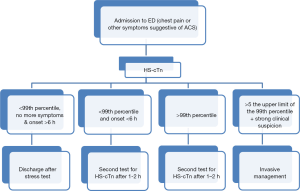
The introduction of the universal definition of MI has led to a major degree of worldwide harmonization in diagnosing ACS (25,43,44), thus contributing to reduce diagnostic inconsistencies. The diagnosis of MI is not only made by measuring cTn, since the risk scores based on anamnesis and clinical presentation along with the information provided by electrocardiography and (possibly) imaging techniques are still crucial to the process. However, clinicians should be aware of the central relevance of cTn testing and shall consider the application of diagnostic protocols that are both up to date and feasible within their work environment (43-46) (Figure 2). It is hence worthwhile mentioning here the recommendations issued in 1999 by the NACB (7):
- Members of EDs, divisions of cardiology, hospital administrations, and clinical laboratories should work collectively to develop an accelerated protocol for the use of biochemical markers in the evaluation of patients with possible acute coronary syndromes (ACS) (Class I);
- For simplicity, this protocol should apply to either the facilitated diagnosis or the rule-out of AMI in the ED or to routine diagnosis from other areas of the hospital, should a patient develop symptoms consistent with ACSs while hospitalized (Class II).
According to the many aspects discussed in the previous section of this article, the organizational advantages of introducing HS-cTn testing in the ED are mostly related to early rule out. This is because the vast majority (80–85%) of patients admitted with suspected ACS and for whom cTn testing is required, end up with a diagnosis different from ischemic heart disease (27). The suitability of ruling out an ACS according to the initial values of cTn has been envisioned in the ESC guidelines already in 2011 (47), subsequently reaffirmed by an Italian “ad hoc” interdisciplinary working group (45), and finally reiterated in the most recent ESC guidelines (25). In all documents it is clearly affirmed that an initial cTn value below the 99th percentile is necessary to possibly rule out an ACS. The exclusion of myocardial ischemia may be confirmed when the cTn value measured with a HS immunoassay remains fairly stable also on repeated testing. Therefore, considering the turnaround time of the currently available techniques, an ACS may be ruled out and eventually patients may be discharged after 3–4 h from admission, at the latest. While this algorithm represents a definite advantage compared to the use of contemporary sensitive cTn assays, the most of which requires a 6-h algorithm for safe rule out of ACS, additional refinements have been suggested. The most recent ESC guidelines (25) indicate that, whenever a HS-cTn immunoassay “with a validated protocol” is available, an accelerated algorithm encompassing shortened testing (i.e., baseline and after 1–2 h) may be seen as a valuable perspective. Recent evidences also suggest that the cTn value at presentation combined with a delta of absolute cTn values should be preferable over the use of the percentage variation that was originally proposed (25,27). This approach would enable faster rule, being characterized by a very high negative predictive value (NPV), ranging from 99.6% (31) to 99.8% (48). Interestingly, shorter sampling with HS-cTn immunoassays were found to have almost equal diagnostic accuracy compared to the “standard” 3 h algorithm (48). The absolute delta increase of cTn values should however be tailored according to the analytical characteristics of the method, thus always higher than the value characterized by 10% imprecision (8). Therefore, standard algorithms need to be developed and validated for each of the potential HS immunoassays that are (or will be) available in the market.
An even faster approach would encompass the use of a single, lower cutoff value for ruling out MI, that will also resolve the debate regarding the appropriateness of using multiple age-adjusted and sex-adjusted thresholds (49). One key point of the 1 h algorithm is that the cutoff is much lower than the 99th percentile, so increasing both sensitivity and NPV. This has been the matter of other studies aimed at identifying better thresholds than the 99th percentile to rule out MI at presentation, prompted by insufficient sensitivity for clinical use of the 99th percentile (50). A lower threshold, set around 5–6 ng/L, has hence been proposed (48,49), as both values are within the 10% total CV of the novel HS-cTnI immunoassay. Shah et al. (51) analyzed a cohort of more than 6,300 consecutive patients and found that 56–61% of patients without MI showed cTnI concentrations <5 ng/L, and the NPV for ruling out MI was as high as 99.4–99.6%, remaining consistent across groups stratified by age, sex, risk factors and previous cardiovascular disease. Carlton et al. (52) studied 3,155 patients presenting with suspect ACS and non-ischemic ECG, showing that the value corresponding to the limit of detection (LoD) as diagnostic threshold yielded a sensitivity as high as 99.0% (95% CI, 96.8–99.7%) and a NPV of 99.5% (95% CI, 98.4–99.9%), allowing early discharge of a considerable number of patients (approximately 20%). The key aspect here may be of interest for the emergency physicians when adopting the different diagnostic options for ruling out ACS by HS-cTn (Table 2).
The organizational advantages that will emerge from these approaches (i.e., accelerating the discharge of patients from the ED), are basically the same. The adoption of specific algorithms and timing for serial sampling should then be planned considering the sensitivity for MI and its diagnostic accuracy for predicting short-term outcomes, but should also consider some environmental factors (i.e., distance between the ED and the laboratory, means of sample transportation, type of health care facility, etc.) and analytic criteria (8).
From an economic perspective, we should first remember that the incremental cost of these protocols is substantially meaningless. The diagnosis of NSTEMI is now almost entirely based on cTn, the total cost of which rarely exceeds $2–4 US (53), i.e., approximately 2-time higher than that of a contemporary sensitive method. On the other side, in a western country like Italy the average cost for each patient admitted to the ED for MI or arrhythmia (a typical yellow/red code) is around $700 US and a single hour of stay costs about approximates $100 US (54,55). The cost emerging from replacing contemporary sensitive with HS techniques would hence be completely overwhelmed by a most efficient diagnosis and a much earlier discharge.
A systematic review and meta-analysis of diagnostic strategies for suspect ACS (56) has revealed that in most scenarios, HS-cTn measurement was the most effective strategy, with an incremental cost-effectiveness ratio (ICER) of less than the £20,000–30,000/QALY (quality-adjusted life years) (ICER £7,487–17,191/QALY). This aspect was further investigated by the UK National Institute for Clinical Excellence (57), reaching rather similar conclusions. Notably, the transferability of these results is limited, since these figures were based on the evidence available at the time of the studies, which were mostly based in the use of the 99th percentile as the decision value. Additional studies are ongoing to establish whether or not the use of short sampling algorithms entailing the use cutoffs lower than the 99th percentile value may be really effective to reducing the overall healthcare cost and enabling a more efficient use of resources in the ED.
Conclusions
Recent evidence suggests that the use of HS-cTn may guarantee better analytical performances and will enable a shift to more rapid rule out strategies (58,59). This is especially true when the decision cutoff is lower than the 99th percentile value. A single, very low value of HS-cTn at presentation may be sufficient to rule out MI with a NPV that is really close to 100%, especially in low-risk patients and in ED settings where a lower prevalence of MI diagnosis is observed in patients presenting with suspect ACS. To achieve a high NPV, the low LoD (i.e., a cTnI value of around 5–6 ng/L) appears to be the safer criterion and will allow to discharge rapidly 20% or more of patients with negative ECG findings with a high accuracy, whereas higher cutoff values will increase this percentage compounded by with a higher risk of false negative results (i.e., non-ischemic injury). Indeed, the time of presentation after symptoms onset is critical and it seems advisable to obtain a second sample after 1–2 h in patients who present earlier or with uncertain timing, as well as in patients with a high risk score (25). The economic benefits that will stem from an accelerated rule-out depend upon the demographics of people admitted to the ED and the health care policies of the different countries and regions. However, considering the relatively low cost of HS-cTn immunoassays, the cost/benefit analysis will predictably generate a favorable scenario. Throughout the history of ACS diagnostics many advancements have make it possible to substantially ameliorate the clinical decision making (60,61) and, indeed, the routine use of HS-cTn immunoassay will represent the next paradigm in this constantly evolving scenario.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Galli is currently employed by Abbott Diagnostics as the Associate Director, Medical Scientific Liaison Europe. Dr. Lippi has no conflicts of interest to declare.
References
- Cummins P, Young A, Auckland ML, et al. Comparison of serum cardiac specific troponin-I with creatine kinase, creatine kinase-MB isoenzyme, tropomyosin, myoglobin and C-reactive protein release in marathon runners: cardiac or skeletal trauma? Eur J Clin Invest 1987;17:317-24. [Crossref] [PubMed]
- Bodor GS, Porter S, Landt Y, et al. Development of monoclonal antibodies for an assay of cardiac troponin-l and preliminary results in suspected cases of myocardial infarction. Clin Chem 1992;38:2203-14. [PubMed]
- Hamm CW, Giannitsis E, Katus HA. Cardiac troponin elevations in patients without acute coronary syndrome. Circulation 2002;106:2871-2. [Crossref] [PubMed]
- Hamm CW, Goldmann BU, Heeschen C, et al. Emergency room triage of patients with acute chest pain by means of rapid testing for cardiac troponin T or troponin I. N Engl J Med 1997;337:1648-53. [Crossref] [PubMed]
- Bertrand ME, Simoons ML, Fox KA, et al. Management of acute coronary syndromes: acute coronary syndromes without persistent ST segment elevation: recommendations of the Task Force of the European Society of Cardiology. Eur Heart J 2000;21:1406-32. [Crossref] [PubMed]
- Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction: executive summary and recommendations: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee on the management of patients with unstable angina). Circulation 2000;102:1193-9. [Crossref] [PubMed]
- Wu AH, Apple FS, Gibler WB, et al. National Academy of Clinical Biochemistry Standards of Laboratory Practice: recommendations for use of cardiac markers in coronary artery diseases. Clin Chem 1999;45:1104-21. [PubMed]
- Lippi G. The mystifying nomenclature of cardiac troponin immunoassays. Scand J Clin Lab Invest 2014;74:273-7. [Crossref] [PubMed]
- Lippi G, Cervellin G. High-sensitivity troponin T is more susceptible than high-sensitivity troponin I to impaired renal function. Am J Cardiol 2013;112:1985. [Crossref] [PubMed]
- Jesse RL. On the relative value of an assay versus that of a test: a history of troponin for the diagnosis of myocardial infarction. J Am Coll Cardiol 2010;55:2125-8. [Crossref] [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, et al. TThird universal definition of myocardial infarction. Circulation 2012;126:2020-35. [Crossref] [PubMed]
- Apple FS. Collinson PO, for the IFCC Task Force on Clinical Applications of Cardiac Biomarkers. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem 2012;58:54-61. [Crossref] [PubMed]
- Apple FS, Ler R, Murakami MM. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clin Chem 2012;58:1574-81. [Crossref] [PubMed]
- Sandoval Y, Apple FS. The global need to define normality: the 99th percentile value of cardiac troponin. Clin Chem 2014;60:455-62. [Crossref] [PubMed]
- Franzini M, Lorenzoni V, Masotti S, et al. The calculation of the cardiac troponin T 99th percentile of the reference population is affected by age, gender, and population selection: A multicenter study in Italy. Clin Chim Acta 2015;438:376-81. [Crossref] [PubMed]
- Krintus M, Kozinski M, Boudry P, et al. Defining normality in a European multinational cohort: Critical factors influencing the 99th percentile upper reference limit for high sensitivity cardiac troponin I. Int J Cardiol 2015;187:256-63. [Crossref] [PubMed]
- Eggers KM, James S, Venge P, et al. Prognostic implications of changes in cardiac troponin I levels in patients with non-ST elevation acute coronary syndrome. Biomarkers 2013;18:668-72. [Crossref] [PubMed]
- Sinning C, Keller T, Zeller T, et al. Association of high-sensitivity assayed troponin I with cardiovascular phenotypes in the general population: the population-based Gutenberg health study. Clin Res Cardiol 2014;103:211-22. [Crossref] [PubMed]
- Omland T, de Lemos JA, Holmen OL, et al. Impact of sex on the prognostic value of high-sensitivity cardiac troponin I in the general population: the HUNT study. Clin Chem 2015;61:646-56. [Crossref] [PubMed]
- Zeller T, Ojeda F, Brunner FJ, et al. High-sensitivity cardiac troponin I in the general population--defining reference populations for the determination of the 99th percentile in the Gutenberg Health Study. Clin Chem Lab Med 2015;53:699-706. [Crossref] [PubMed]
- Koerbin G, Potter JM, Abhayaratna WP, et al. Longitudinal studies of cardiac troponin I in a large cohort of healthy children. Clin Chem 2012;58:1665-72. [Crossref] [PubMed]
- Caselli C, Cangemi G, Masotti S, et al. Plasma cardiac troponin I concentrations in healthy neonates, children and adolescents measured with a high sensitive immunoassay method. Clin Chim Acta 2016;458:68-71. [Crossref] [PubMed]
- Conrad MJ, Jarolim P. Cardiac troponins and high sensitivity cardiac troponin assays. Clin Lab Med 2014;34:59-73. [Crossref] [PubMed]
- Gamble JH, Carlton EW, Orr WP, et al. High-sensitivity cardiac troponins: no more ‘negatives’. Expert Rev Cardiovasc Ther 2013;11:1129-39. [Crossref] [PubMed]
- Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2016;37:267-315. [Crossref] [PubMed]
- Pines JM, Pollack CV Jr, Diercks DB, et al. The association between emergency department crowding and adverse cardiovascular outcomes in patients with chest pain. Acad Emerg Med 2009;16:617-25. [Crossref] [PubMed]
- Keller T, Zeller T, Ojeda F, et al. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA 2011;306:2684-93. [Crossref] [PubMed]
- Goodacre S, Cross E, Arnold J, et al. The health care burden of acute chest pain. Heart 2005;91:229-30. [Crossref] [PubMed]
- Montalescot G, Crea F. The year in cardiology 2015: acute coronary syndromes. Eur Heart J 2016;37:221-8. [Crossref] [PubMed]
- Bhuiya FA, Pitts SR, McCaig LF. Emergency department visits for chest pain and abdominal pain: United States, 1999–2008. NCHS Data Brief 2010;43:1-8. [PubMed]
- Rubini Gimenez M, Twerenbold R, Jaeger C, et al. One-hour rule-in and rule-out of acute myocardial infarction using high-sensitivity cardiac troponin I. Am J Med 2015;128:861-870.e4. [Crossref] [PubMed]
- Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med 2009;16:1-10. [Crossref] [PubMed]
- Wildi K, Gimenez MR, Twerenbold R, et al. Misdiagnosis of myocardial infarction related to limitations of the current regulatory approach to define clinical decision values for cardiac troponin. Circulation 2015;131:2032-40. [Crossref] [PubMed]
- Gan SC, Beaver SK, Houck PM, et al. Treatment of acute myocardial infarction and 30-day mortality among women and men. N Engl J Med 2000;343:8-15. [Crossref] [PubMed]
- Vaccarino V, Rathore SS, Wenger NK, et al. Sex and racial differences in the management of acute myocardial infarction, 1994 through 2002. N Engl J Med 2005;353:671-82. [Crossref] [PubMed]
- EUGenMed Cardiovascular Clinical Study Group, Regitz-Zagrosek V, Oertelt-Prigione S, et al. Gender in cardiovascular diseases: impact on clinical manifestations, management, and outcomes. Eur Heart J 2016;37:24-34. [Crossref] [PubMed]
- Mehta LS, Beckie TM, DeVon HA, et al. Acute myocardial infarction in women. A scientific statement from the American Heart Association. Circulation 2016;133:916-47. [Crossref] [PubMed]
- Cervellin G, Rastelli G. The clinics of acute coronary syndrome. Ann Transl Med 2016;4:191. [Crossref] [PubMed]
- Lippi G, Sanchis-Gomar F, Cervellin G. Chest pain, dyspnea and other symptoms in patients with type 1 and 2 myocardial infarction. A literature review. Int J Cardiol 2016;215:20-2. [Crossref] [PubMed]
- Shah AS, Griffiths M, Lee KK, et al. High sensitivity cardiac troponin and the under-diagnosis of myocardial infarction in women: prospective cohort study. BMJ 2015;350:g7873. [Crossref] [PubMed]
- Lindsell CJ, Anantharaman V, Diercks D, et al. The Internet tracking registry of acute coronary syndromes (i*trACS): a multicenter registry of patients with suspicion of acute coronary syndromes reported using the standardized reporting guidelines for emergency department chest pain studies. Ann Emerg Med 2006;48:666-77, 677.e1-9. [Crossref] [PubMed]
- Bohula May EA, Bonaca MP, Jarolim P, et al. Prognostic performance of a high-sensitivity cardiac troponin I assay in patients with non–ST-elevation acute coronary syndrome. Clin Chem 2014;60:158-64. [Crossref] [PubMed]
- Thygesen K, Mair J, Giannitsis E, et al. The Study Group on Biomarkers in Cardiology of the ESC Working Group on Acute Cardiac Care. How to use high-sensitivity cardiac troponins in acute cardiac care. Eur Heart J 2012;33:2252-7. [Crossref] [PubMed]
- Apple FS, Jaffe AS, Collinson P, et al. IFCC educational materials on selected analytical and clinical applications of high sensitivity cardiac troponin assays. Clin Biochem 2015;48:201-3. [Crossref] [PubMed]
- Casagranda I, Cavazza M, Clerico A, et al. Proposal for the use in emergency departments of cardiac troponins measured with the latest generation methods in patients with suspected acute coronary syndrome without persistent ST-segment elevation. Clin Chem Lab Med 2013;51:1727-37. [Crossref] [PubMed]
- Cervellin G, Mattiuzzi C, Bovo C, et al. Diagnostic algorithms for acute coronary syndrome-is one better than another? Ann Transl Med 2016;4:193. [Crossref] [PubMed]
- Hamm CW, Bassand JP, Agewall S, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2011;32:2999-3054. [Crossref] [PubMed]
- Neumann JT, Sörensen NA, Schwemer T, et al. Diagnosis of myocardial infarction using a high-sensitivity troponin I 1-hour algorithm. JAMA Cardiol 2016. [Epub ahead of print].
- Lippi G. Novel troponin immunoassay for early ACS rule-out Nat Rev Cardiol 2016;13:9-10. [Crossref] [PubMed]
- Pickering JW, Greenslade JH, Cullen L, et al. Validation of presentation and 3 h high-sensitivity troponin to rule-in and rule-out acute myocardial infarction. Heart 2016. [Epub ahead of print]. [PubMed]
- Shah AS, Anand A, Sandoval Y, et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet 2015;386:2481-8. [Crossref] [PubMed]
- Carlton E, Greenslade J, Cullen L, et al. Evaluation of high-sensitivity cardiac troponin I levels in patients with suspected acute coronary syndrome. JAMA Cardiol 2016. [Epub ahead of print].
- Lackner KJ. High sensitivity assays for cardiac troponins. Clin Chem Lab Med 2015;53:631-3. [Crossref] [PubMed]
- Guasticchi G and working group. Methodological proposal for the cost evaluation in emergency medicine. Italian Health Ministry, Progetto Mattoni SSN 2007.
- Cremonesi P, Di Bella E, Montefiori M. Cost analysis of emergency department. J Prev Med Hyg 2010;51:157-63. [PubMed]
- Goodacre S, Thokala P, Carroll C, et al. Systematic review, meta-analysis and economic modelling of diagnostic strategies for suspected acute coronary syndrome. Health Technol Assess 2013;17:v-vi, 1-188. [Crossref] [PubMed]
- National Institute for Health and Care Excellence (NICE). Myocardial infarction (acute): Early rule out using high-sensitivity troponin tests (Elecsys Troponin T high-sensitive, ARCHITECT STAT High Sensitive Troponin-I and AccuTnI+3 assays). Diagnostics guidance, 2014.
- Lippi G, Cervellin G. Acute coronary syndrome: many doubts, some answers. Ann Transl Med 2016;4:187. [Crossref] [PubMed]
- Danese E, Montagnana M. An historical approach to the diagnostic biomarkers of acute coronary syndrome. Ann Transl Med 2016;4:194. [Crossref] [PubMed]
- Lippi G, Montagnana M, Salvagno GL, et al. Potential value for new diagnostic markers in the early recognition of acute coronary syndromes. CJEM 2006;8:27-31. [PubMed]
- Cervellin G, Lippi G. Of MIs and men--a historical perspective on the diagnostics of acute myocardial infarction. Semin Thromb Hemost 2014;40:535-43. [Crossref] [PubMed]